Conserved immuno-collagenic subtypes predict response to immune checkpoint blockade
Abstract
Background
Immune checkpoint blockade (ICB) has revolutionized the treatment of various cancer types. Despite significant preclinical advancements in understanding mechanisms, identifying the molecular basis and predictive biomarkers for clinical ICB responses remains challenging. Recent evidence, both preclinical and clinical, underscores the pivotal role of the extracellular matrix (ECM) in modulating immune cell infiltration and behaviors. This study aimed to create an innovative classifier that leverages ECM characteristics to enhance the effectiveness of ICB therapy.
Methods
We analyzed transcriptomic collagen activity and immune signatures in 649 patients with cancer undergoing ICB therapy. This analysis led to the identification of three distinct immuno-collagenic subtypes predictive of ICB responses. We validated these subtypes using the transcriptome data from 9,363 cancer patients from The Cancer Genome Atlas (TCGA) dataset and 1,084 in-house samples. Additionally, novel therapeutic targets were identified based on these established immuno-collagenic subtypes.
Results
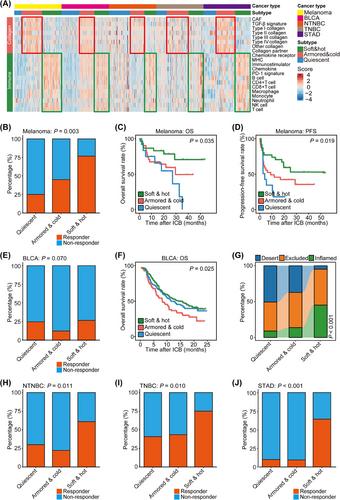
Our categorization divided tumors into three subtypes: “soft & hot” (low collagen activity and high immune infiltration), “armored & cold” (high collagen activity and low immune infiltration), and “quiescent” (low collagen activity and immune infiltration). Notably, “soft & hot” tumors exhibited the most robust response to ICB therapy across various cancer types. Mechanistically, inhibiting collagen augmented the response to ICB in preclinical models. Furthermore, these subtypes demonstrated associations with immune activity and prognostic predictive potential across multiple cancer types. Additionally, an unbiased approach identified B7 homolog 3 (B7-H3), an available drug target, as strongly expressed in “armored & cold” tumors, relating with poor prognosis.
Conclusion
This study introduces histopathology-based universal immuno-collagenic subtypes capable of predicting ICB responses across diverse cancer types. These findings offer insights that could contribute to tailoring personalized immunotherapeutic strategies for patients with cancer.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


