Claudin 18.2 as a novel therapeutic target
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
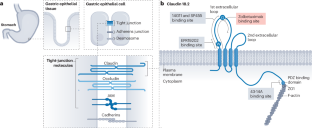
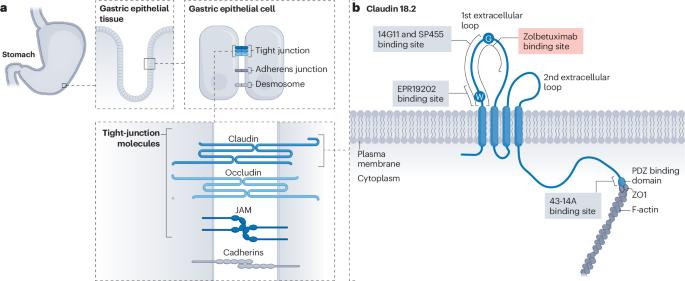
Claudin 18.2, a tight-junction molecule predominantly found in the nonmalignant gastric epithelium, becomes accessible on the tumour cell surface during malignant transformation, thereby providing an appealing target for cancer therapy. Data from two phase III trials testing the anti-claudin 18.2 antibody zolbetuximab have established claudin 18.2-positive advanced-stage gastric cancers as an independent therapeutic subset that derives benefit from the addition of this agent to chemotherapy. This development has substantially increased the percentage of patients eligible for targeted therapy. Furthermore, newer treatments, such as high-affinity monoclonal antibodies, bispecific antibodies, chimeric antigen receptor T cells and antibody–drug conjugates capable of bystander killing effects, have shown considerable promise in patients with claudin 18.2-expressing gastric cancers. This new development has resulted from drug developers moving beyond traditional targets, such as driver gene alterations or growth factors. In this Review, we highlight the biological rationale and explore the clinical activity of therapies that target claudin 18.2 in patients with advanced-stage gastric cancer and explore the potential for expansion of claudin 18.2-targeted therapies to patients with other claudin 18.2-positive solid tumours. The development and successful phase III testing of the anti-claudin 18.2 antibody zolbetuximab has provided a novel targeted therapy for the 30–40% of patients with strongly claudin 18.2-positive gastric cancers. Furthermore, the development of an effective targeted therapy for a target that does not have a driver role in cancer development provides a novel drug development paradigm. In this Review, the authors describe the development of claudin 18.2-targeted therapies, including zolbetuximab, as well as novel therapies, including chimeric antigen receptor (CAR) T cells, antibody–drug conjugates and bispecific antibodies, all of which have the potential to expand the number of patients who can derive benefit from claudin 18.2-targeted therapies in the near future.
作为新型治疗靶点的 Claudin 18.2
克劳丁 18.2 是一种紧密连接分子,主要存在于非恶性胃上皮细胞中,在恶性转化过程中可进入肿瘤细胞表面,从而为癌症治疗提供了一个有吸引力的靶点。抗克劳丁 18.2 抗体唑贝妥昔单抗(zolbetuximab)的两项 III 期试验数据表明,克劳丁 18.2 阳性晚期胃癌是一个独立的治疗亚群,在化疗的基础上加用这种药物可使其获益。这一进展大大增加了符合靶向治疗条件的患者比例。此外,高亲和力单克隆抗体、双特异性抗体、嵌合抗原受体 T 细胞和具有旁观者杀伤效应的抗体-药物共轭物等新疗法已在表达 claudin 18.2 的胃癌患者中大显身手。这一新发展是药物开发人员超越传统靶点(如驱动基因改变或生长因子)的结果。在这篇综述中,我们将重点介绍针对晚期胃癌患者的claudin 18.2靶向疗法的生物学原理和临床活性,并探讨将claudin 18.2靶向疗法扩展到其他claudin 18.2阳性实体瘤患者的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


