Availability and stock-outs of paediatric antiretroviral treatment formulations at health facilities in Kenya and Uganda
Abstract
Introduction
The large number of deaths among children with HIV is driven by poor antiretroviral treatment (ART) coverage among this cohort. The aim of the study was to assess the availability and stock-outs of paediatric and adult ART formulations in Kenya and Uganda across various regions and types of health facilities.
Methods
A survey on availability and stock-outs of paediatric ART at health facilities was adapted from the standardized Health Action International–WHO Medicine Availability Monitoring Tool. All preferred and limited-use formulations, and three phased-out formulations according to the 2021 WHO optimal formulary list were included in the survey, as well as a selection of adult ART formulations suitable for older children, adolescents, and adults. Availability data were collected in June–July 2022 and stock-out data were obtained over the previous year from randomly selected public and private-not-for-profit (PNFP) facilities registered to dispense paediatric ART across six districts per country. All data were analysed descriptively.
Results
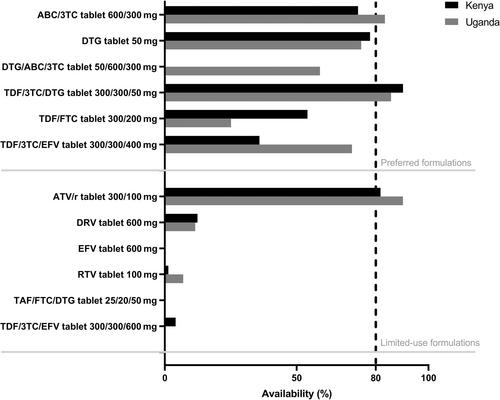
In total, 144 health facilities were included (72 per country); 110 were public and 34 PNFP facilities. Overall availabilities of preferred paediatric ART formulations were 52.2% and 63.5% in Kenya and Uganda, respectively, with dolutegravir (DTG) 10 mg dispersible tablets being available in 70.2% and 77.4% of facilities, respectively, and abacavir/lamivudine dispersible tablets in 89.8% and 98.2% of facilities. Of note, availability of both formulations was low (37.5% and 62.5%, respectively) in Kenyan PNFP facilities. Overall availabilities of paediatric limited-use products were 1.1% in Kenya and 1.9% in Uganda. At least one stock-out of a preferred paediatric ART formulation was reported in 40.0% of Kenyan and 74.7% of Ugandan facilities. Nevirapine solution stock-outs were reported in 43.1% of Ugandan facilities, while alternative formulations for postnatal HIV prophylaxis were not available.
Conclusions
Recommended DTG-based first-line ART for children across all ages was reasonably available at health facilities in Kenya and Uganda, with the exception of Kenyan PNFP facilities. Availability of paediatric ART formulations on the limited-use list was extremely low across both countries. Stock-outs were reported regularly, with the high number of reported stock-outs of neonatal ART formulations in Uganda being most concerning.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


