Simulation of avascular tumor growth and drug response in a microfluidic device with a cellular automaton model
Abstract
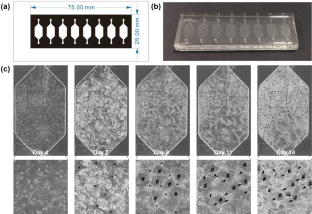
The microfluidic system is capable of recapitulating key attributes of in vivo circumstances and, therefore, becomes a valuable platform for better understanding tumor growth dynamics and evaluating drug efficiency. While numerical simulations have been envisioned as powerful tools for validating versatile performance of advanced microfluidic platforms, cell growth within these microchannels has not yet been theoretically modeled. In this paper, we developed an experimental data-driven cellular automaton model, which was adopted for simulating cell behaviors and drug responses in a microfluidic system. The boundaries of the cellular automata lattices and prohibited zones for simulation were directly converted from microscopic images of cell morphology and the microchamber configuration. The dynamic progression of tumor growth at the avascular stage was predicted by incorporating the biophysical and molecular characteristics of cells and their interactions with surrounding environment. The simulated proliferation rate of tumor cells over time demonstrated its dependency on nutrient delivery, aligning well with experimental observations in the microfluidic culture. The spatiotemporal efficacy of the chemotherapeutic compound doxorubicin (DOX) on the microfluidic culture was also simulated. The similarity between in silico simulations and in vitro tumor response upon drug interaction highlighted the potential of the computational models as complementary tools for predicting the drug treatment efficacy with acceptable accuracy before practical applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


