Adsorptive Removal of \({{\text{UO}}}_{2}^{2+}\) Ions from Aqueous Solutions by Ni(II)/Fe(III)-Layered Double Hydroxides and its Magnetic Nanocomposites
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
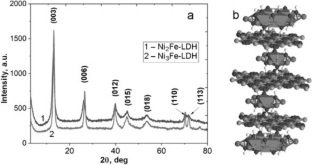
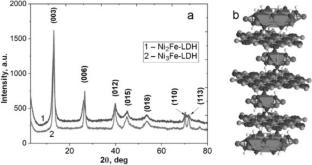
Comparative analysis of the sorption ability of the obtained carbonate forms of layered double hydroxides with different Ni(II)/Fe(III) ratio and their magnetic nanocomposites toward \({{\text{UO}}}_{2}^{2+}\) ions in aqueous solutions is performed. The synergistic effect of magnetic composite sorbents based on layered double hydroxides and magnetite on the removal uranyl ions in a wide pH range (3.5-9.5) is shown. Due to the high efficiency in combination with magnetic solid-phase separation, the obtained adsorption materials are promising for the purification of uranium-containing natural and wastewaters.
镍(II)/铁(III)层状双氢氧化物及其磁性纳米复合材料吸附去除水溶液中的 $${{text{UO}}}_{2}^{2+}$ 离子
比较分析了所获得的不同镍(II)/铁(III)比的碳酸盐形式的层状双氢氧化物及其磁性纳米复合材料对水溶液中\({text{UO}}}_{2}^{2+}\)离子的吸附能力。结果表明,在较宽的 pH 值范围(3.5-9.5)内,基于层状双氢氧化物和磁铁矿的磁性复合吸附剂对铀酰离子的去除具有协同效应。由于与磁性固相分离相结合具有很高的效率,所获得的吸附材料有望用于净化含铀的天然水和废水。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


