A self-amplifying loop of TP53INP1 and P53 drives oxidative stress-induced apoptosis of bone marrow mesenchymal stem cells
Abstract
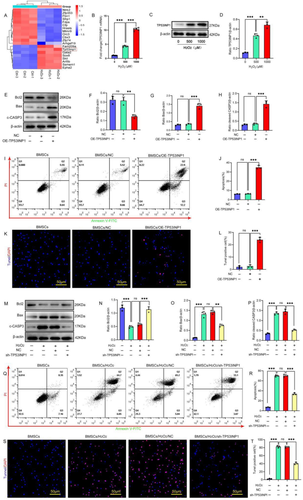
Bone marrow mesenchymal stem cell (BMSC) transplantation is a promising regenerative therapy; however, the survival rate of BMSCs after transplantation is low. Oxidative stress is one of the main reasons for the high apoptosis rate of BMSCs after transplantation, so there is an urgent need to explore the mechanism of oxidative stress-induced apoptosis of BMSCs. Our previous transcriptome sequencing results suggested that the expression of P53-induced nuclear protein 1 (TP53INP1) and the tumor suppressor P53 (P53) was significantly upregulated during the process of oxidative stress-induced apoptosis of BMSCs. The present study further revealed the role and mechanism of TP53INP1 and P53 in oxidative stress-induced apoptosis in BMSCs. Overexpression of TP53INP1 induced apoptosis of BMSCs, knockdown of TP53INP1 alleviated oxidative stress apoptosis of BMSCs. Under oxidative stress conditions, P53 is regulated by TP53INP1, while P53 can positively regulate the expression of TP53INP1, so the two form a positive feedback loop. To clarify the mechanism of feedback loop formation. We found that TP53INP1 inhibited the ubiquitination and degradation of P53 by increasing the phosphorylation level of P53, leading to the accumulation of P53 protein. P53 can act on the promoter of the TP53INP1 gene and increase the expression of TP53INP1 through transcriptional activation. This is the first report on a positive feedback loop formed by TP53INP1 and P53 under oxidative stress. The present study clarified the formation mechanism of the positive feedback loop. The TP53INP1–P53 positive feedback loop may serve as a potential target for inhibiting oxidative stress-induced apoptosis in BMSCs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


