Gut bacteria–derived serotonin promotes immune tolerance in early life
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
The gut microbiota promotes immune system development in early life, but the interactions between the gut metabolome and immune cells in the neonatal gut remain largely undefined. Here, we demonstrate that the neonatal gut is uniquely enriched with neurotransmitters, including serotonin, and that specific gut bacteria directly produce serotonin while down-regulating monoamine oxidase A to limit serotonin breakdown. We found that serotonin directly signals to T cells to increase intracellular indole-3-acetaldehdye and inhibit mTOR activation, thereby promoting the differentiation of regulatory T cells, both ex vivo and in vivo in the neonatal intestine. Oral gavage of serotonin into neonatal mice resulted in long-term T cell–mediated antigen-specific immune tolerance toward both dietary antigens and commensal bacteria. Together, our study has uncovered an important role for specific gut bacteria to increase serotonin availability in the neonatal gut and identified a function of gut serotonin in shaping T cell response to dietary antigens and commensal bacteria to promote immune tolerance in early life.
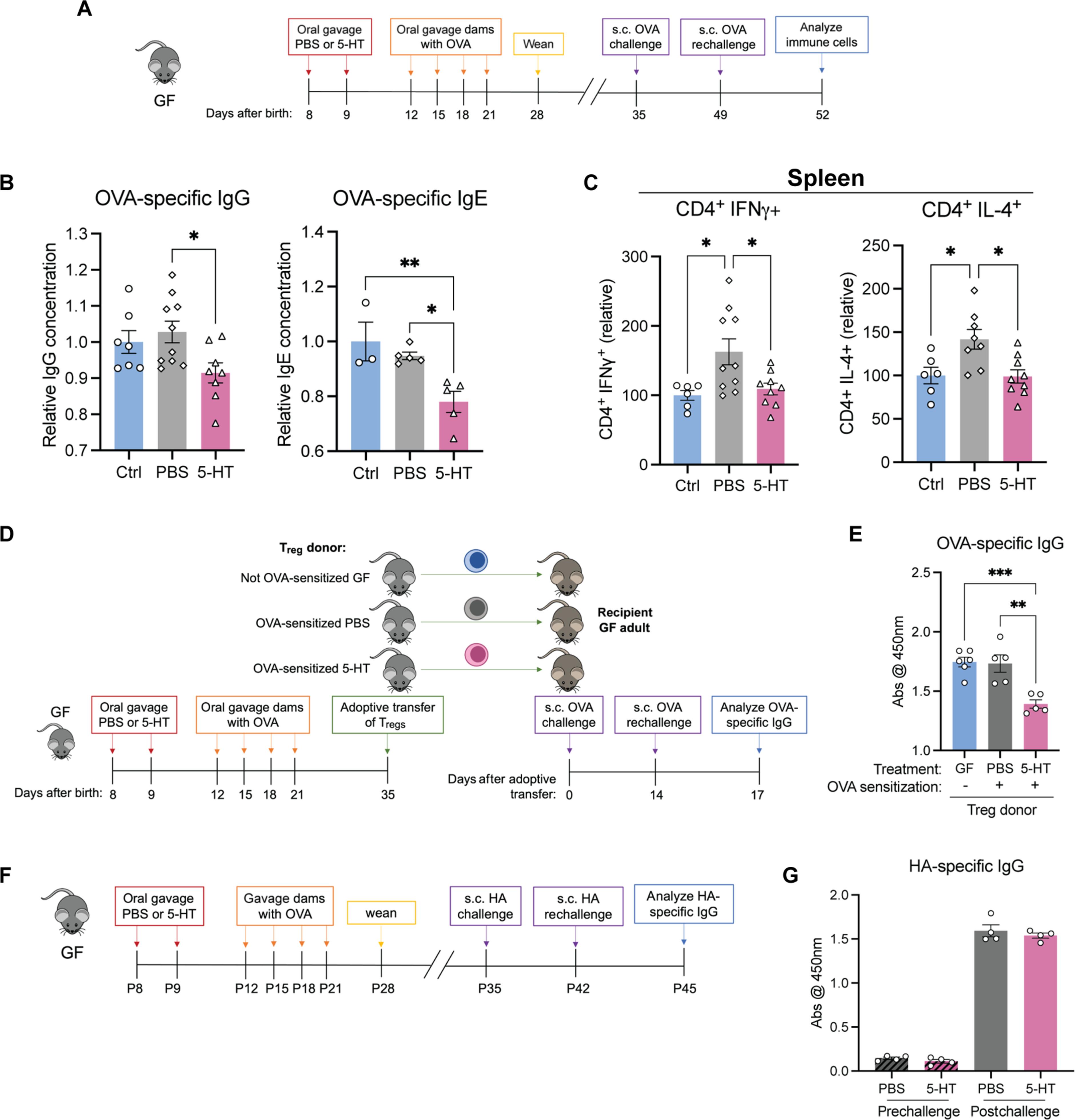
源自肠道细菌的血清素可促进生命早期的免疫耐受。
肠道微生物群促进生命早期免疫系统的发育,但新生儿肠道代谢组和免疫细胞之间的相互作用在很大程度上仍未确定。在这里,我们证明了新生儿肠道独特地富含包括血清素在内的神经递质,特定的肠道细菌直接产生血清素,同时下调单胺氧化酶 A 以限制血清素的分解。我们发现,5-羟色胺直接向T细胞发出信号,增加细胞内吲哚-3-乙酰胆碱染料,抑制mTOR的激活,从而促进调节性T细胞的分化。给新生小鼠口服羟色胺可导致T细胞介导的对饮食抗原和共生细菌的长期抗原特异性免疫耐受。总之,我们的研究揭示了特定肠道细菌在增加新生儿肠道血清素可用性方面的重要作用,并确定了肠道血清素在塑造 T 细胞对饮食抗原和共生细菌的反应以促进生命早期免疫耐受方面的功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


