Synthetic Approaches to the New Drugs Approved During 2022
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
In 2022, 23 new small molecule chemical entities were approved as drugs by the United States FDA, European Union EMA, Japan PMDA, and China NMPA. This review describes the synthetic approach demonstrated on largest scale for each new drug based on patent or primary literature. The synthetic routes highlight practical methods to construct molecules, sometimes on the manufacturing scale, to access the new drugs. Ten additional drugs approved in 2021 and one approved in 2020 are included that were not covered in the previous year’s review.
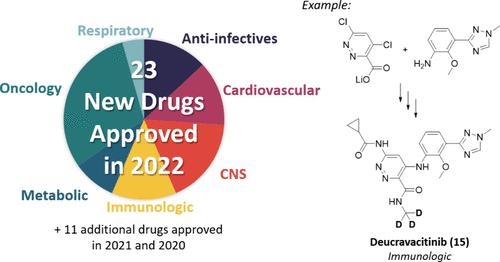
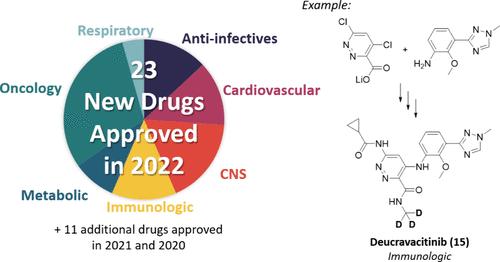
2022 年期间获批新药的合成方法。
2022 年,23 种新的小分子化学实体被美国 FDA、欧盟 EMA、日本 PMDA 和中国 NMPA 批准为药物。本综述介绍了根据专利或原始文献为每种新药论证的最大规模合成方法。合成路线重点介绍了构建分子的实用方法,有时是在生产规模上构建分子,以获得新药。本综述还收录了 2021 年批准的 10 种新药和 2020 年批准的 1 种新药,这些新药在上一年的综述中均未涉及。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


