Smoking and outcomes following personalized antiplatelet therapy in chronic coronary syndrome patients: A substudy from the randomized PATH-PCI trial
Abstract
Background
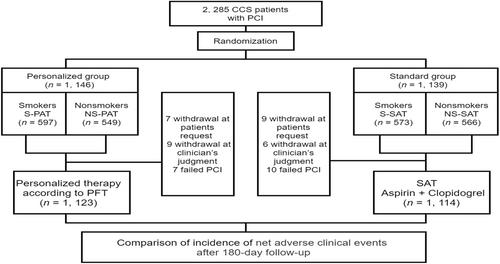
This is a sub-analysis of the Personalized Antithrombotic Therapy for Coronary Heart Disease after PCI (PATH-PCI) trial in China to explore the relationship between smoking and outcomes following personalized antiplatelet therapy (PAT) in chronic coronary syndrome (CCS) patients undergoing percutaneous coronary intervention (PCI).
Methods
As a single-center, prospective, randomized controlled and open-label trial, the PATH-PCI trial randomized CCS patients undergoing PCI into standard group or personalized group guided by a novel platelet function test (PFT), from December 2016 to February 2018. All patients were divided into smokers and nonsmokers according to their smoking status. Subsequently, we underwent a 180-day follow-up evaluation. The primary endpoint was the net adverse clinical events (NACE).
Results
Regardless of smoking status, in the incidence of NACE, there was a reduction with PAT but that the reductions are not statistically significant. In the incidence of bleeding events, we found no statistically significant difference between two groups (smokers: 2.0% vs. 1.4%, HR = 1.455, 95% confidence interval [CI]: 0.595−3.559, p = .412; nonsmokers: 2.2% vs. 1.8%, HR = 1.228, 95% CI: 0.530−2.842, p = .632). In smokers, PAT reduced major adverse cardiac and cerebrovascular events (MACCE) by 48.7% (3.0% vs. 5.9%, HR = 0.513, 95% CI: 0.290−0.908, p = .022), compared with standard antiplatelet therapy (SAT). PAT also reduced the major adverse cardiovascular events (MACE) but there was no statistically difference in the reductions (p > .05). In nonsmokers, PAT reduced MACCE and MACE by 51.5% (3.3% vs. 6.7%, HR = 0.485, 95% CI: 0.277−0.849, p = .011) and 63.5% (1.8% vs. 4.9%, HR = 0.365, 95% CI: 0.178−0.752, p = .006), respectively. When testing p-values for interaction, we found there was no significant interaction of smoking status with treatment effects of PAT (pint-NACE = .184, pint-bleeding = .660).
Conclusion
Regardless of smoking, PAT reduced the MACE and MACCE, with no significant difference in bleeding. This suggests that PAT was an recommendable regimen to CCS patients after PCI, taking into consideration both ischemic and bleeding risk.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


