Backbone 1H, 13C and 15N resonance assignment of the ubiquitin specific protease 7 catalytic domain (residues 208–554) in complex with a small molecule ligand
IF 0.6
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
Abstract
The backbone 1H, 13C and 15N resonance assignment of Ubiquitin Specific Protease 7 catalytic domain (residues 208–554) was performed in its complex with a small molecule ligand and in its apo form as a reference. The amide 1H-15N signal intensities were boosted by an amide hydrogen exchange protocol, where expressed 2H, 13C, 15N-labeled protein was unfolded and re-folded to ensure exchange of amide deuterons to protons. The resonance assignments were used to determine chemical shift perturbations on ligand binding, which are consistent with the binding site observed by crystallography.
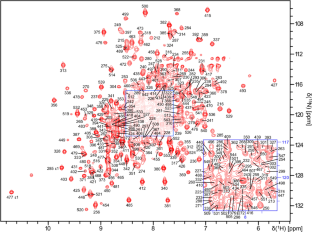
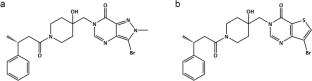
泛素特异性蛋白酶 7 催化结构域(残基 208-554)与小分子配体复合物的骨架 1H、13C 和 15N 共振赋值。
在泛素特异性蛋白酶 7 催化结构域(残基 208-554)与小分子配体的复合物中,以及作为参照物的apo形式中,对其进行了骨架 1H、13C 和 15N 共振赋值。通过酰胺氢交换方案提高了酰胺 1H-15N 信号强度,在该方案中,将表达的 2H、13C、15N 标记蛋白质展开并重新折叠,以确保酰胺氘核交换为质子。共振分配用于确定配体结合时的化学位移扰动,这与晶体学观察到的结合位点一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


