Adjudicative efficacy of Bifidobacterium animalis subsp. lactis BLa80 in treating acute diarrhea in children: a randomized, double-blinded, placebo-controlled study
IF 3.6
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
Abstract
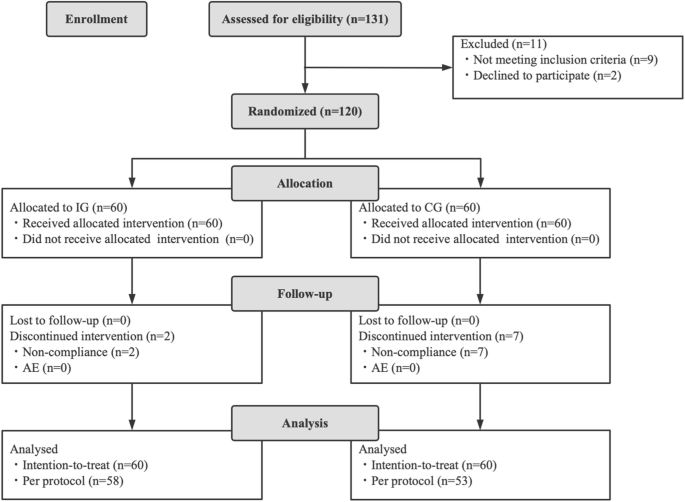
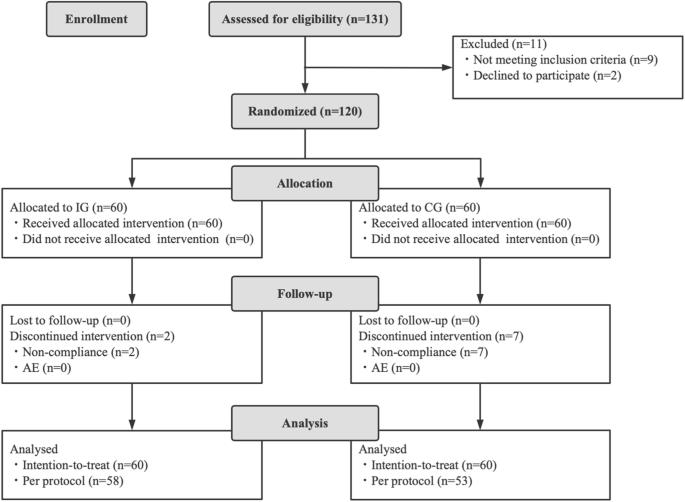
The goal of this study is to assess the efficacy and safety of Bifidobacterium animalis subsp. lactis BLa80, as an adjunct treatment for diarrhea in children with a randomized, double-blinded, placebo-controlled study design. Eligible diarrheal children, aged 0–3 years without the need for antibiotic treatment based on clinical diagnosis when recruited, were randomized into the intervention group (IG, n = 58, with probiotic) or the control group (CG, n = 53, placebo). The primary assessment was the duration of diarrhea. Fecal samples were collected for biochemical index measurement, analysis of gut microbiome composition, and prediction of gene family abundances. The total duration of diarrhea in the IG (122.6 ± 13.1 h) was significantly shorter than in the CG (148.4 ± 17.6 h, p < 0.001). More children in the IG showed improvements in diarrhea compared to the CG, both in intention-to-treat analysis (81.7% vs. 40.0%, p < 0.001) and per protocol analysis (84.4% vs 45.3%, p < 0.001). Cathelicidin level in the IG was significantly higher than that in the CG after the intervention (4415.00 ± 1036.93 pg/g vs. 3679.49 ± 871.18 pg/g, p = 0.0175). The intervention led to an increased abundance of Bifidobacterium breve and Collinsella aerofaciens species, higher alpha-diversity (p < 0.05), and enrichment of functional genes in the gut microbiota related to immunity regulation. Administration of BLa80 at a dose of 5 × 109 CFU/day resulted in a shorter duration of diarrhea and alterations in gut microbiome composition and gene functions.
动物双歧杆菌亚种乳杆菌 BLa80 治疗儿童急性腹泻的疗效判断:一项随机、双盲、安慰剂对照研究
本研究旨在通过随机、双盲、安慰剂对照研究设计,评估动物双歧杆菌亚种乳杆菌 BLa80 作为儿童腹泻辅助治疗药物的有效性和安全性。根据临床诊断,符合条件的 0-3 岁腹泻儿童在招募时不需要抗生素治疗,他们被随机分为干预组(IG,n = 58,使用益生菌)或对照组(CG,n = 53,使用安慰剂)。主要评估指标是腹泻持续时间。收集粪便样本用于生化指标测定、肠道微生物组组成分析和基因家族丰度预测。IG 的总腹泻持续时间(122.6 ± 13.1 小时)明显短于 CG(148.4 ± 17.6 小时,p < 0.001)。在意向治疗分析(81.7% vs. 40.0%,p < 0.001)和按方案分析(84.4% vs. 45.3%,p < 0.001)中,IG 组比 CG 组有更多患儿的腹泻情况有所改善。干预后,IG 中的柔毛素水平明显高于 CG(4415.00 ± 1036.93 pg/g vs. 3679.49 ± 871.18 pg/g,p = 0.0175)。干预措施增加了布氏双歧杆菌和科林斯菌的数量,提高了α-多样性(p < 0.05),并丰富了肠道微生物群中与免疫调节相关的功能基因。以 5 × 109 CFU/天的剂量服用 BLa80 可缩短腹泻持续时间,并改变肠道微生物组的组成和基因功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.60
自引率
2.10%
发文量
189
审稿时长
3-6 weeks
期刊介绍:
The European Journal of Clinical Nutrition (EJCN) is an international, peer-reviewed journal covering all aspects of human and clinical nutrition. The journal welcomes original research, reviews, case reports and brief communications based on clinical, metabolic and epidemiological studies that describe methodologies, mechanisms, associations and benefits of nutritional interventions for clinical disease and health promotion.
Topics of interest include but are not limited to:
Nutrition and Health (including climate and ecological aspects)
Metabolism & Metabolomics
Genomics and personalized strategies in nutrition
Nutrition during the early life cycle
Health issues and nutrition in the elderly
Phenotyping in clinical nutrition
Nutrition in acute and chronic diseases
The double burden of ''malnutrition'': Under-nutrition and Obesity
Prevention of Non Communicable Diseases (NCD)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


