Continuous Flow Electroselenocyclization of Allylamides and Unsaturated Oximes to Selenofunctionalized Oxazolines and Isoxazolines
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
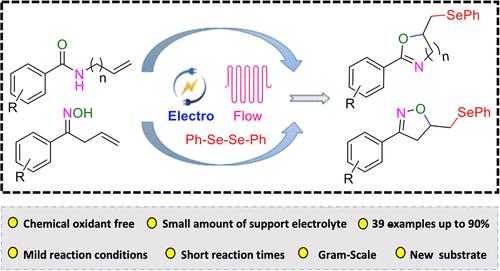
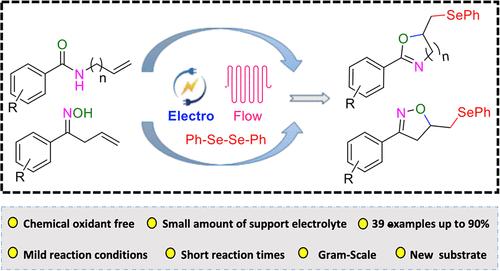
The synthesis of selenofunctionalized oxazolines and isoxazolines from N-allyl benzamides and unsaturated oximes with diselenides was studied by utilizing a continuous flow electrochemical approach. At mild reaction conditions and short reaction times of 10 min product yields of up to 90% were achieved including a scale-up reaction. A broad substrate scope was studied and the reaction was shown to have a wide functional group tolerance.
烯丙基酰胺和不饱和肟与硒官能化噁唑啉和异噁唑啉的连续流电硒环化反应
研究人员利用连续流电化学方法,从 N-烯丙基苯甲酰胺和不饱和肟与二硒化物合成硒官能化噁唑啉和异噁唑啉。在温和的反应条件下和 10 分钟的短反应时间内,包括放大反应在内,产品收率高达 90%。研究了广泛的底物范围,结果表明该反应具有广泛的官能团耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


