求助PDF
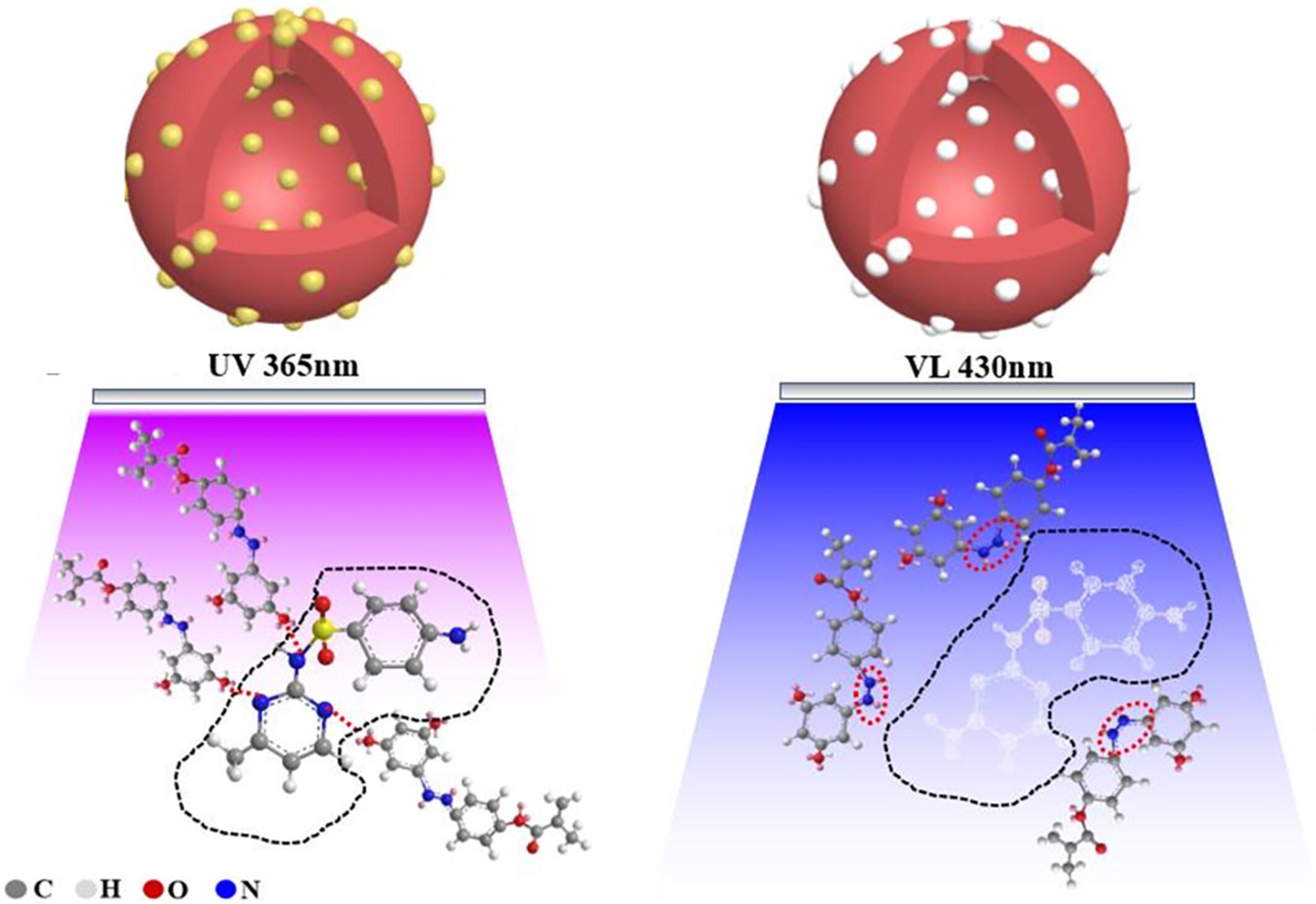
{"title":"Novel photoresponsive molecularly imprinted polymers based on etched silicon core with enabling enhanced selectivity and sensitivity for the detection of sulfamethazine","authors":"Wangui Peng, Weihong Huang, Minmin Gao, Wenming Yang, Wanzhen Xu","doi":"10.1002/pi.6628","DOIUrl":null,"url":null,"abstract":"<p>Sulfamethymidine, a commonly employed sulfonamide, is introduced into the human body through the food chain, posing a threat to human health. Consequently, the development of a rapid and efficient detection technique is imperative. In this investigation, a photoresponsive sulfamethyridiazine-imprinted polymer was synthesized to etch a silicon core, thereby effectively addressing the limitations encountered with conventional molecularly imprinted polymers including diminished binding capacity, restricted site accessibility and sluggish binding kinetics. The photosensitive monomer utilized in the molecularly imprinted polymers is 5[(4 (methylacryloxy) phenyl) diazene] isophthalic acid, which exhibits a stimulating reaction mechanism. The N=N bond undergoes photoisomerization, transitioning between <i>trans</i> and <i>cis</i> configurations. The adsorption experiment provides additional evidence that the hollow molecularly imprinted polymers exhibit a higher adsorption capacity, achieving a value of 0.192 mmol L<sup>−1</sup>. The experiments conducted to assess selectivity and repeatability confirm that the photoresponsive molecularly imprinted polymers exhibit a high level of selectivity and favorable repeatability. Following four cycles, the adsorption rate remains consistently at 64.3%. Additionally, the recoveries of the actual samples ranged from 95.6% to 99.7%. This finding presents a novel approach to detecting the concentration of trace pollutants in intricate substrates. © 2024 Society of Industrial Chemistry.</p>","PeriodicalId":20404,"journal":{"name":"Polymer International","volume":"73 7","pages":"556-562"},"PeriodicalIF":2.9000,"publicationDate":"2024-03-07","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Polymer International","FirstCategoryId":"92","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/pi.6628","RegionNum":4,"RegionCategory":"化学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"POLYMER SCIENCE","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Sulfamethymidine, a commonly employed sulfonamide, is introduced into the human body through the food chain, posing a threat to human health. Consequently, the development of a rapid and efficient detection technique is imperative. In this investigation, a photoresponsive sulfamethyridiazine-imprinted polymer was synthesized to etch a silicon core, thereby effectively addressing the limitations encountered with conventional molecularly imprinted polymers including diminished binding capacity, restricted site accessibility and sluggish binding kinetics. The photosensitive monomer utilized in the molecularly imprinted polymers is 5[(4 (methylacryloxy) phenyl) diazene] isophthalic acid, which exhibits a stimulating reaction mechanism. The N=N bond undergoes photoisomerization, transitioning between trans and cis configurations. The adsorption experiment provides additional evidence that the hollow molecularly imprinted polymers exhibit a higher adsorption capacity, achieving a value of 0.192 mmol L−1 . The experiments conducted to assess selectivity and repeatability confirm that the photoresponsive molecularly imprinted polymers exhibit a high level of selectivity and favorable repeatability. Following four cycles, the adsorption rate remains consistently at 64.3%. Additionally, the recoveries of the actual samples ranged from 95.6% to 99.7%. This finding presents a novel approach to detecting the concentration of trace pollutants in intricate substrates. © 2024 Society of Industrial Chemistry.
一种基于蚀刻硅核的新型光致分子印迹聚合物,可提高磺胺甲基嘧啶检测的选择性和灵敏度
磺胺甲基嘧啶是一种常用的磺胺类药物,通过食物链进入人体,对人类健康构成威胁。因此,开发一种快速高效的检测技术势在必行。在这项研究中,我们合成了一种光致发光的磺胺甲基嘧啶印迹聚合物,可蚀刻硅芯,从而有效解决了传统分子印迹聚合物的局限性,包括结合能力减弱、位点可及性受限和结合动力学迟缓等问题。分子印迹聚合物(MIPs)中使用的光敏单体是 5[(4(甲基丙烯酰氧基)苯基)重氮]邻苯二甲酸,它具有刺激反应机制。N=N 键发生光异构化,在反式和顺式构型之间转换。吸附实验进一步证明,中空分子印迹聚合物具有更高的吸附能力,吸附值达到 0.192 mmol L-1。为评估选择性和可重复性而进行的实验证实,光致发光分子印迹聚合物具有较高的选择性和良好的可重复性。经过四个循环后,吸附率始终保持在 64.3%。经过四个循环后,吸附率始终保持在 64.3%。此外,实际样品的回收率在 95.6% 到 99.7% 之间。这一发现为检测复杂基质中的痕量污染物浓度提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


