Electrocatalysis at vegetable oil water interface
IF 4.7
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
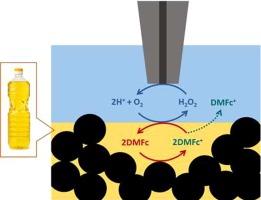
Biphasic oxygen reduction and hydrogen evolution are studied for almost two decades, because of favourable overpotential decrease as compared to aqueous solution. Until now, polar solvents (ε > 7) were employed as organic phase in these studies. Here, we applied non polar vegetable oils (rapeseed, linen or sunflower) for biphasic H2O2 generation by oxygen reduction. This product was detected at oil|aqueous acid solution interface by scanning electrochemical microscopy, when electron donor – decamethylferrocene, was electrochemically recycled. Ejection of small fraction of decamethylferrocenium cation from oil to aqueous phase was also noticed.
植物油水界面的电催化
与水溶液相比,双相氧还原和氢进化具有有利的过电位下降特性,因此对双相氧还原和氢进化的研究已有近二十年的历史。迄今为止,这些研究一直使用极性溶剂(ε > 7)作为有机相。在这里,我们使用非极性植物油(菜籽油、亚麻油或向日葵油)通过氧还原生成双相 H2O2。当电子供体--十甲基二茂铁被电化学回收时,通过扫描电化学显微镜可在油/水酸溶液界面检测到这种产物。此外,还发现一小部分十甲基二茂铁阳离子从油相喷射到水相。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


