Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
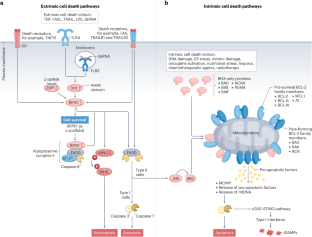
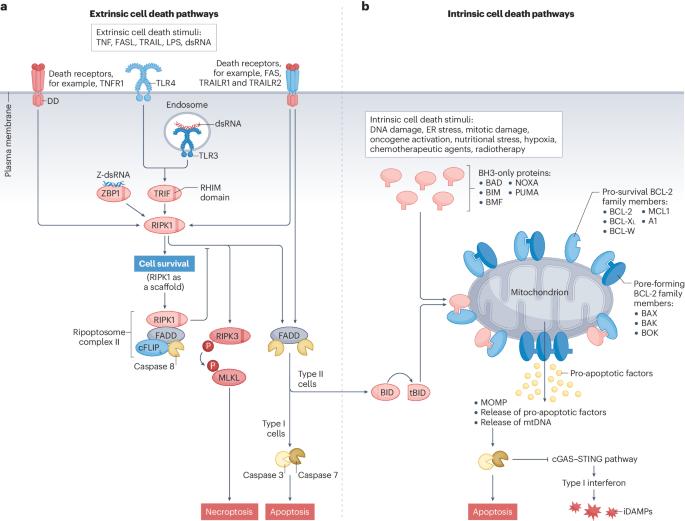
Most metastatic cancers remain incurable due to the emergence of apoptosis-resistant clones, fuelled by intratumour heterogeneity and tumour evolution. To improve treatment, therapies should not only kill cancer cells but also activate the immune system against the tumour to eliminate any residual cancer cells that survive treatment. While current cancer therapies rely heavily on apoptosis — a largely immunologically silent form of cell death — there is growing interest in harnessing immunogenic forms of cell death such as necroptosis. Unlike apoptosis, necroptosis generates second messengers that act on immune cells in the tumour microenvironment, alerting them of danger. This lytic form of cell death optimizes the provision of antigens and adjuvanticity for immune cells, potentially boosting anticancer treatment approaches by combining cellular suicide and immune response approaches. In this Review, we discuss the mechanisms of necroptosis and how it activates antigen-presenting cells, drives cross-priming of CD8+ T cells and induces antitumour immune responses. We also examine the opportunities and potential drawbacks of such strategies for exposing cancer cells to immunological attacks. In this Review, Meier et al. discuss the molecular mechanisms of necroptosis, delineate how this form of immunogenic cell death activates antitumour immune responses and explore the opportunities and limitations of targeting necroptosis for anticancer therapy.
癌症中的免疫性细胞死亡:针对坏死诱导抗肿瘤免疫。
大多数转移性癌症仍然无法治愈,原因是在肿瘤内异质性和肿瘤进化的推动下,出现了抗凋亡克隆。为了改善治疗,疗法不仅要杀死癌细胞,还要激活免疫系统对抗肿瘤,以消灭治疗后存活下来的残余癌细胞。目前的癌症疗法在很大程度上依赖细胞凋亡--一种在免疫学上基本无声的细胞死亡形式--但人们对利用细胞坏死等免疫原性细胞死亡形式的兴趣与日俱增。与细胞凋亡不同,坏死凋亡会产生第二信使,作用于肿瘤微环境中的免疫细胞,向它们发出危险警报。这种细胞死亡的溶解形式优化了免疫细胞抗原和佐剂的提供,通过结合细胞自杀和免疫反应方法,有可能促进抗癌治疗方法。在这篇综述中,我们将讨论坏死的机制,以及它如何激活抗原递呈细胞、驱动 CD8+ T 细胞交叉免疫和诱导抗肿瘤免疫反应。我们还探讨了让癌细胞暴露于免疫攻击的这种策略的机遇和潜在缺点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


