Low resting metabolic rate and increased hunger due to β-MSH and β-endorphin deletion in a canine model
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
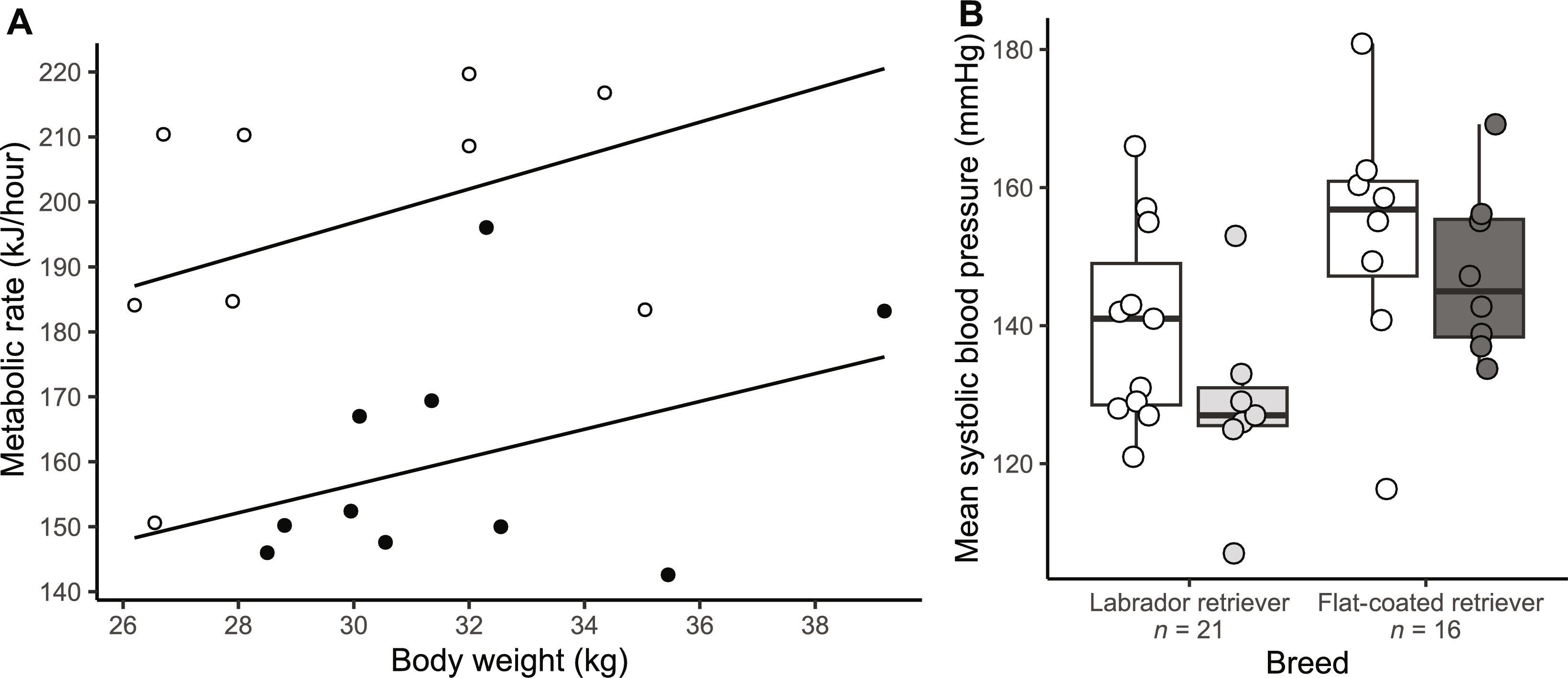
Mutations that perturb leptin-melanocortin signaling are known to cause hyperphagia and obesity, but energy expenditure has not been well studied outside rodents. We report on a common canine mutation in pro-opiomelanocortin (POMC), which prevents production of β–melanocyte-stimulating hormone (β-MSH) and β-endorphin but not α-MSH; humans, similar to dogs, produce α-MSH and β-MSH from the POMC propeptide, but rodents produce only α-MSH. We show that energy expenditure is markedly lower in affected dogs, which also have increased motivational salience in response to a food cue, indicating increased wanting or hunger. There was no difference in satiety at a modified ad libitum meal or in their hedonic response to food, nor disruption of adrenocorticotropic hormone (ACTH) or thyroid axes. In vitro, we show that β-MSH signals comparably to α-MSH at melanocortin receptors. These data implicate β-MSH and β-endorphin as important in determining hunger and moderating energy expenditure and suggest that this role is independent of the presence of α-MSH.
在犬模型中,β-MSH 和 β-内啡肽缺失会导致静息代谢率降低和饥饿感增强。
众所周知,扰乱瘦素-黑色素皮质素信号传导的突变可导致食欲亢进和肥胖,但对啮齿类动物以外的能量消耗还没有进行过深入研究。我们报告了一种常见的犬前黑皮促皮质素(POMC)突变,这种突变会阻止β-黑皮细胞刺激素(β-MSH)和β-内啡肽的产生,但不会阻止α-MSH的产生;人类与狗类似,会从POMC的前肽产生α-MSH和β-MSH,但啮齿类动物只产生α-MSH。我们的研究表明,受影响的狗的能量消耗明显降低,它们对食物线索的动机显著性也增加了,这表明它们的欲望或饥饿感增加了。在改良的自由进餐中,它们的饱腹感或对食物的享乐反应没有差异,促肾上腺皮质激素(ACTH)或甲状腺轴也没有受到破坏。在体外,我们发现在黑皮质素受体上,β-MSH 的信号与 α-MSH 相当。这些数据表明,β-MSH 和 β-内啡肽在决定饥饿感和调节能量消耗方面起着重要作用,并表明这种作用与 α-MSH 的存在无关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


