Guiding the HBO1 complex function through the JADE subunit
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
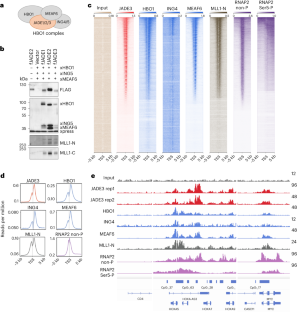
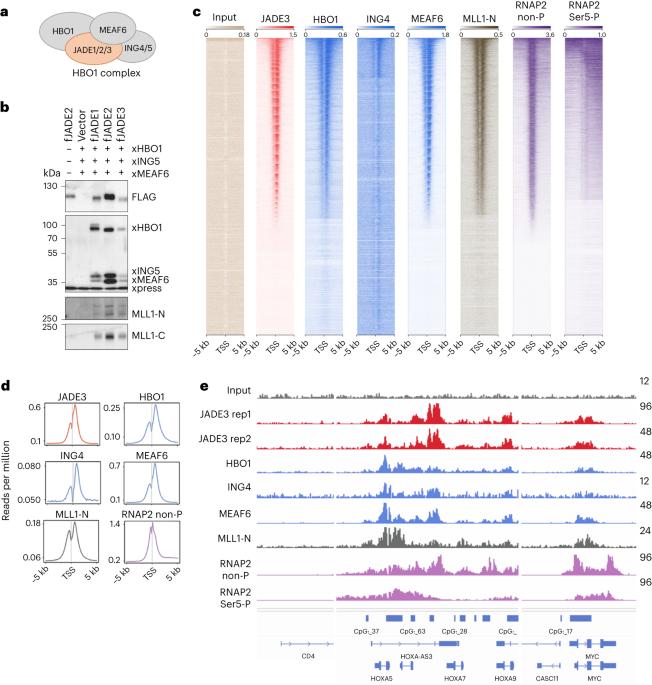
JADE is a core subunit of the HBO1 acetyltransferase complex that regulates developmental and epigenetic programs and promotes gene transcription. Here we describe the mechanism by which JADE facilitates recruitment of the HBO1 complex to chromatin and mediates its enzymatic activity. Structural, genomic and complex assembly in vivo studies show that the PZP (PHD1–zinc-knuckle–PHD2) domain of JADE engages the nucleosome through binding to histone H3 and DNA and is necessary for the association with chromatin targets. Recognition of unmethylated H3K4 by PZP directs enzymatic activity of the complex toward histone H4 acetylation, whereas H3K4 hypermethylation alters histone substrate selectivity. We demonstrate that PZP contributes to leukemogenesis, augmenting transforming activity of the NUP98–JADE2 fusion. Our findings highlight biological consequences and the impact of the intact JADE subunit on genomic recruitment, enzymatic function and pathological activity of the HBO1 complex. JADE is a subunit of human acetyltransferase complex HBO1 that is essential in transcriptional regulation. Gaurav et al. characterize the molecular mechanism by which JADE mediates genomic association and enzymatic and pathological activities of the HBO1 complex.
通过 JADE 亚基引导 HBO1 复合物的功能。
JADE是HBO1乙酰转移酶复合物的核心亚基,该复合物调控发育和表观遗传程序并促进基因转录。在这里,我们描述了 JADE 促进 HBO1 复合物招募到染色质并介导其酶活性的机制。结构、基因组和体内复合物组装研究表明,JADE 的 PZP(PHD1-锌扣-PHD2)结构域通过与组蛋白 H3 和 DNA 结合而与核小体结合,并且是与染色质靶标结合的必要条件。PZP 能识别未甲基化的 H3K4,将复合体的酶活性导向组蛋白 H4 乙酰化,而 H3K4 过度甲基化则会改变组蛋白底物的选择性。我们证明,PZP 能增强 NUP98-JADE2 融合体的转化活性,从而促进白血病的发生。我们的研究结果突显了完整的 JADE 亚基对 HBO1 复合物的基因组招募、酶功能和病理活性的生物学后果和影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


