DNA-functionalized artificial mechanoreceptor for de novo force-responsive signaling
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Synthetic signaling receptors enable programmable cellular responses coupling with customized inputs. However, engineering a designer force-sensing receptor to rewire mechanotransduction remains largely unexplored. Herein, we introduce nongenetically engineered artificial mechanoreceptors (AMRs) capable of reprogramming non-mechanoresponsive receptor tyrosine kinases (RTKs) to sense user-defined force cues, enabling de novo-designed mechanotransduction. AMR is a modular DNA–protein chimera comprising a mechanosensing-and-transmitting DNA nanodevice grafted on natural RTKs via aptameric anchors. AMR senses intercellular tensile force via an allosteric DNA mechano-switch with tunable piconewton-sensitive force tolerance, actuating a force-triggered dynamic DNA assembly to manipulate RTK dimerization and activate intracellular signaling. By swapping the force-reception ligands, we demonstrate the AMR-mediated activation of c-Met, a representative RTK, in response to the cellular tensile forces mediated by cell-adhesion proteins (integrin, E-cadherin) or membrane protein endocytosis (CI-M6PR). Moreover, AMR also allows the reprogramming of FGFR1, another RTK, to customize mechanobiological function, for example, adhesion-mediated neural stem cell maintenance. Yang et al. reported the development of nongenetically engineered artificial mechanoreceptors capable of reprogramming non-mechanoresponsive receptors to sense user-defined force cues, enabling de novo-designed mechanotransduction.
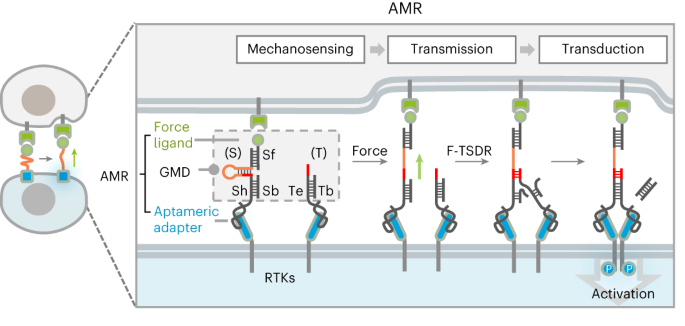
DNA 功能化人工机械感受器,用于新的力响应信号传导。
合成信号受体可实现与定制输入耦合的可编程细胞反应。然而,对设计的力感应受体进行工程化以重新连接机械传导在很大程度上仍未得到探索。在这里,我们介绍了非遗传工程人造机械感受器(AMR),它能够重新编程非机械反应性受体酪氨酸激酶(RTK),使其能够感知用户定义的力线索,从而实现全新设计的机械传导。AMR 是一种模块化的 DNA 蛋白嵌合体,由机械传感和传输 DNA 纳米器件组成,通过适配体锚接枝到天然 RTK 上。AMR 通过一个具有可调皮牛顿力耐受性的异构 DNA 机械开关来感知细胞间的拉力,从而驱动一个由力触发的动态 DNA 组件来操纵 RTK 的二聚化并激活细胞内的信号传导。通过交换力接收配体,我们展示了 AMR 介导的 c-Met 激活(一种代表性 RTK),以响应由细胞粘附蛋白(整合素、E-cadherin)或膜蛋白内吞(CI-M6PR)介导的细胞拉力。此外,AMR 还能使另一种 RTK FGFR1 重新编程,以定制机械生物学功能,例如粘附介导的神经干细胞维护。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
文献相关原料
公司名称
产品信息
阿拉丁
Blebbistatin
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


