Role of biophysics and mechanobiology in podocyte physiology
IF 28.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Podocytes form the backbone of the glomerular filtration barrier and are exposed to various mechanical forces throughout the lifetime of an individual. The highly dynamic biomechanical environment of the glomerular capillaries greatly influences the cell biology of podocytes and their pathophysiology. Throughout the past two decades, a holistic picture of podocyte cell biology has emerged, highlighting mechanobiological signalling pathways, cytoskeletal dynamics and cellular adhesion as key determinants of biomechanical resilience in podocytes. This biomechanical resilience is essential for the physiological function of podocytes, including the formation and maintenance of the glomerular filtration barrier. Podocytes integrate diverse biomechanical stimuli from their environment and adapt their biophysical properties accordingly. However, perturbations in biomechanical cues or the underlying podocyte mechanobiology can lead to glomerular dysfunction with severe clinical consequences, including proteinuria and glomerulosclerosis. As our mechanistic understanding of podocyte mechanobiology and its role in the pathogenesis of glomerular disease increases, new targets for podocyte-specific therapeutics will emerge. Treating glomerular diseases by targeting podocyte mechanobiology might improve therapeutic precision and efficacy, with potential to reduce the burden of chronic kidney disease on individuals and health-care systems alike. In this Review, the authors examine the biophysical and biomechanical properties that influence podocyte physiology as they integrate and adapt to stimuli from their dynamic environment within the glomerular capillaries. The authors also discuss how dysregulation and loss of biomechanical resilience in podocytes can contribute to kidney disease.
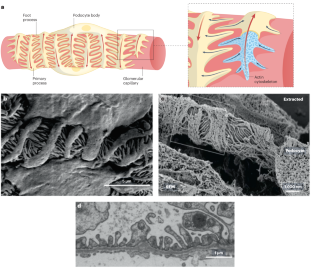
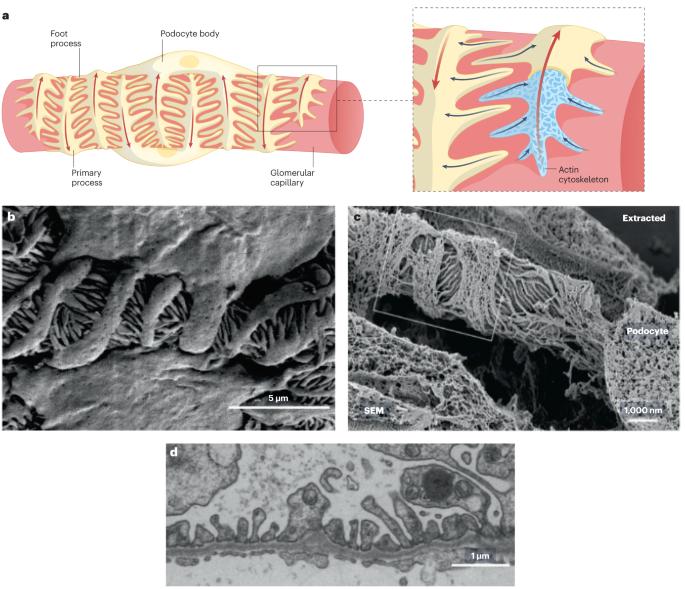
生物物理学和机械生物学在荚膜细胞生理学中的作用。
荚膜细胞是肾小球滤过屏障的支柱,在人的一生中会受到各种机械力的作用。肾小球毛细血管高度动态的生物力学环境极大地影响了荚膜细胞的细胞生物学及其病理生理学。在过去的二十年里,人们对荚膜细胞生物学有了一个全面的认识,强调机械生物学信号通路、细胞骨架动力学和细胞粘附力是决定荚膜细胞生物力学复原力的关键因素。这种生物力学弹性对荚膜细胞的生理功能至关重要,包括形成和维持肾小球滤过屏障。荚膜细胞会整合来自周围环境的各种生物力学刺激,并相应地调整其生物物理特性。然而,生物力学线索或潜在的荚膜细胞机械生物学的干扰会导致肾小球功能障碍,造成严重的临床后果,包括蛋白尿和肾小球硬化。随着我们对荚膜细胞机械生物学及其在肾小球疾病发病机制中作用的机理认识的加深,荚膜细胞特异性疗法的新靶点将会出现。通过针对荚膜细胞机械生物学来治疗肾小球疾病可能会提高治疗的精确性和疗效,从而有可能减轻慢性肾病给个人和医疗系统带来的负担。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


