Altered purinergic P2X7 and A2B receptors signaling limits macrophage-mediated host defense in schistosomiasis
IF 4.1
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
The occurrence of co-infections during schistosomiasis, a neglected tropical disease, with other parasites have been reported suggesting an impaired host immune defense. Macrophage purinergic P2X7 receptor (P2X7R) plays an important role against intracellular pathogens. Therefore, we investigated the P2X7R-mediated phagocytosis and killing capacity of Leishmania amazonensis by macrophages during schistosomiasis in vitro and in vivo.
Methods
Swiss and C57BL/6 (Wild type) and P2X7R−/− were randomized in two groups: control (uninfected) and Schistosoma mansoni-infected. Alternatively, control Swiss and S. mansoni-infected mice were also infected with L. amazonensis.
Results
The pre-treatment of control macrophages with the P2X7R antagonist (A74003) or TGF-β reduced the phagocytosis index, mimicking the phenotype of cells from S. mansoni-infected mice and P2X7R−/− mice. Apyrase also reduced the phagocytosis index in the control group corroborating the role of ATP to macrophage activation. Moreover, l-arginine-nitric oxide pathway was compromised during schistosomiasis, which could explain the reduced killing capacity in response to ATP in vitro and in vivo. We found an increased extracellular nucleotide (ATP, ADP and AMP) hydrolysis along with an increased frequency of F4/80+ CD39+ macrophages from the S. mansoni-infected group. Moreover, the content of adenosine in the cell supernatant was higher in the S. mansoni-infected group in relation to controls. Schistosomiasis also increased the expression of macrophage adenosine A2BR. In good accordance, both ADA and the selective A2BR antagonist restored the phagocytosis index of macrophages from S. mansoni-infected group.
Conclusions
Altogether, the altered P2X7R and A2BR signaling limits the role of macrophages to host defense against L. amazonensis during schistosomiasis, potentially contributing to the pathophysiology and clinically relevant co-infections.
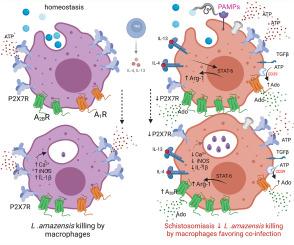
嘌呤能 P2X7 和 A2B 受体信号的改变限制了血吸虫病中巨噬细胞介导的宿主防御。
背景:血吸虫病是一种被忽视的热带疾病,有报道称血吸虫病与其他寄生虫同时感染,这表明宿主的免疫防御功能受损。巨噬细胞嘌呤能 P2X7 受体(P2X7R)在对抗细胞内病原体方面发挥着重要作用。因此,我们研究了血吸虫病期间巨噬细胞在体外和体内介导的 P2X7R 对亚马逊利什曼原虫的吞噬和杀灭能力:方法:将瑞士和 C57BL/6(野生型)以及 P2X7R-/- 随机分为两组:对照组(未感染)和感染曼氏血吸虫组。另外,对照组瑞士小鼠和感染曼氏血吸虫的小鼠也感染了亚马逊血吸虫:结果:用P2X7R拮抗剂(A74003)或TGF-β预处理巨噬细胞可降低吞噬指数,模拟曼氏血吸虫感染小鼠和P2X7R-/-小鼠细胞的表型。Apyrase也降低了吞噬指数,证实了ATP对巨噬细胞活化的作用。此外,L-精氨酸-一氧化氮通路受损,这可以解释体外和体内对 ATP 的杀伤能力降低的原因。我们发现,曼森氏杆菌感染组细胞外核苷酸(ATP、ADP和AMP)水解增加,F4/80+ CD39+巨噬细胞的频率增加。此外,与对照组相比,曼森氏杆菌感染组细胞上清液中的腺苷含量更高。血吸虫病也增加了巨噬细胞腺苷 A2BR 的表达。ADA和选择性A2BR拮抗剂能很好地恢复曼氏血吸虫病感染组巨噬细胞的吞噬指数:总之,P2X7R 和 A2BR 信号的改变限制了血吸虫病期间巨噬细胞在宿主防御亚马逊嗜血杆菌中的作用,可能会导致病理生理学和临床相关的合并感染。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomedical Journal
Medicine-General Medicine
CiteScore
11.60
自引率
1.80%
发文量
128
审稿时长
42 days
期刊介绍:
Biomedical Journal publishes 6 peer-reviewed issues per year in all fields of clinical and biomedical sciences for an internationally diverse authorship. Unlike most open access journals, which are free to readers but not authors, Biomedical Journal does not charge for subscription, submission, processing or publication of manuscripts, nor for color reproduction of photographs.
Clinical studies, accounts of clinical trials, biomarker studies, and characterization of human pathogens are within the scope of the journal, as well as basic studies in model species such as Escherichia coli, Caenorhabditis elegans, Drosophila melanogaster, and Mus musculus revealing the function of molecules, cells, and tissues relevant for human health. However, articles on other species can be published if they contribute to our understanding of basic mechanisms of biology.
A highly-cited international editorial board assures timely publication of manuscripts. Reviews on recent progress in biomedical sciences are commissioned by the editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


