Cytoskeletal β-tubulin and cysteine cathepsin L deregulation by SARS-CoV-2 spike protein interaction with the neuronal model cell line SH-SY5Y
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
SARS-CoV-2 mainly infects the respiratory tract but can also target other organs, including the central nervous system. While it was recently shown that cells of the blood-brain-barrier are permissive to SARS-CoV-2 infection in vitro, it remains debated whether neurons can be infected. In this study, we demonstrate that vesicular stomatitis virus particles pseudotyped with the spike protein of SARS-CoV-2 variants WT, Alpha, Delta and Omicron enter the neuronal model cell line SH-SY5Y. Cell biological analyses of the pseudo-virus treated cultures showed marked alterations in microtubules of SH-SY5Y cells. Because the changes in β-tubulin occurred in most cells, but only few were infected, we further asked whether interaction of the cells with spike protein might be sufficient to cause molecular and structural changes. For this, SH-SY5Y cells were incubated with trimeric spike proteins for time intervals of up to 24 h. CellProfiler™-based image analyses revealed changes in the intensities of microtubule staining in spike protein-incubated cells. Furthermore, expression of the spike protein-processing protease cathepsin L was found to be up-regulated by wild type, Alpha and Delta spike protein pseudotypes and cathepsin L was found to be secreted from spike protein-treated cells. We conclude that the mere interaction of the SARS-CoV-2 with neuronal cells can affect cellular architecture and proteolytic capacities. The molecular mechanisms underlying SARS-CoV-2 spike protein induced cytoskeletal changes in neuronal cells remain elusive and require future studies.
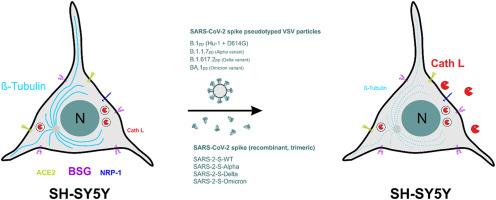
SARS-CoV-2尖峰蛋白与神经元模型细胞株SH-SY5Y相互作用导致细胞骨架β-微管蛋白和半胱氨酸酪蛋白酶L失调。
SARS-CoV-2 主要感染呼吸道,但也可能感染其他器官,包括中枢神经系统。虽然最近有研究表明,血脑屏障细胞在体外对 SARS-CoV-2 感染是允许的,但神经元是否会受到感染仍存在争议。在这项研究中,我们证明了以 SARS-CoV-2 变体 WT、Alpha、Delta 和 Omicron 的尖峰蛋白为伪型的水泡性口炎病毒颗粒能进入神经元模型细胞株 SH-SY5Y。对经过伪病毒处理的培养物进行的细胞生物学分析表明,SH-SY5Y 细胞的微管发生了明显变化。由于β-微管蛋白的变化发生在大多数细胞中,但只有少数细胞受到感染,我们进一步询问细胞与尖峰蛋白的相互作用是否足以引起分子和结构的变化。为此,我们将 SH-SY5Y 细胞与三聚尖峰蛋白孵育了 24 小时。基于 CellProfiler™ 的图像分析显示了尖峰蛋白培养细胞中微管染色强度的变化。此外,我们还发现野生型、α和δ尖峰蛋白假型上调了尖峰蛋白加工蛋白酶cathepsin L的表达,并发现尖峰蛋白处理过的细胞分泌了cathepsin L。我们的结论是,SARS-CoV-2 与神经细胞的相互作用就能影响细胞结构和蛋白水解能力。SARS-CoV-2尖峰蛋白诱导神经元细胞的细胞骨架变化的分子机制仍然难以捉摸,需要今后进行研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimie
生物-生化与分子生物学
CiteScore
7.20
自引率
2.60%
发文量
219
审稿时长
40 days
期刊介绍:
Biochimie publishes original research articles, short communications, review articles, graphical reviews, mini-reviews, and hypotheses in the broad areas of biology, including biochemistry, enzymology, molecular and cell biology, metabolic regulation, genetics, immunology, microbiology, structural biology, genomics, proteomics, and molecular mechanisms of disease. Biochimie publishes exclusively in English.
Articles are subject to peer review, and must satisfy the requirements of originality, high scientific integrity and general interest to a broad range of readers. Submissions that are judged to be of sound scientific and technical quality but do not fully satisfy the requirements for publication in Biochimie may benefit from a transfer service to a more suitable journal within the same subject area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


