SARS-CoV-2 testing, positivity, and factors associated with COVID-19 among people with HIV across Europe in the multinational EuroSIDA cohort
Abstract
Background
Although people with HIV might be at risk of severe outcomes from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; coronavirus 2019 [COVID-19]), regional and temporal differences in SARS-CoV-2 testing in people with HIV across Europe have not been previously described.
Methods
We described the proportions of testing, positive test results, and hospitalizations due to COVID-19 between 1 January 2020 and 31 December 2021 in the EuroSIDA cohort and the factors associated with being tested for SARS-CoV-2 and with ever testing positive.
Results
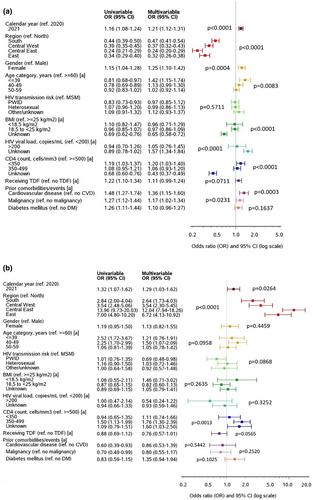
Of 9012 participants, 2270 (25.2%, 95% confidence interval [CI] 24.3–26.1) had a SARS-CoV-2 polymerase chain reaction test during the study period (range: 38.3% in Northern to 14.6% in Central-Eastern Europe). People from Northern Europe, women, those aged <40 years, those with CD4 cell count <350 cells/mm3, and those with previous cardiovascular disease or malignancy were significantly more likely to have been tested, as were people with HIV in 2021 compared with those in 2020. Overall, 390 people with HIV (4.3%, 95% CI 3.9–4.8) tested positive (range: 2.6% in Northern to 7.1% in Southern Europe), and the odds of testing positive were higher in all regions than in Northern Europe and in 2021 than in 2020. In total, 64 people with HIV (0.7%, 95% CI 0.6–0.9) were hospitalized, of whom 12 died. Compared with 2020, the odds of positive testing decreased in all regions in 2021, and the associations with cardiovascular disease, malignancy, and use of tenofovir disoproxil fumarate disappeared in 2021. Among study participants, 58.9% received a COVID-19 vaccine (range: 72.0% in Southern to 14.8% in Eastern Europe).
Conclusions
We observed large heterogeneity in SARS-CoV-2 testing and positivity and a low proportion of hospital admissions and deaths across the regions of Europe.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


