Synthesis of 3,4-Disubstituted Pyrroline Nitroxides Containing Diphenylphosphane or Diphenylphosphane Oxide Substituents
IF 2.1
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
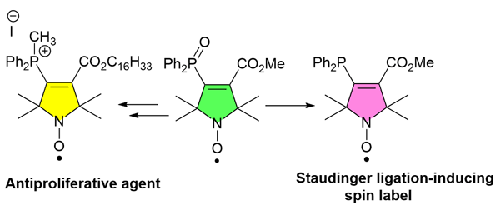
(Methyl 4-(diphenylphosphoryl)-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrole-3-carboxylate-1-yl)oxydanyl was obtained as a key intermediate of the reaction starting from 3,4-dibromo-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-yloxydanyl or (methyl 2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrole-3-carboxylate-1-yl)oxidanyl. This key compound could be converted into an azido-specific Staudinger ligation-inducing spin label, amino- and thiol-specific spin label, or MITO-CP-like antiproliferative agent.
含二苯基膦或二苯基膦氧化物取代基的 3,4-二取代吡咯啉氮氧化物的合成
(4- (二苯基磷酰)-2,2,5,5-四甲基-2,5-二氢-1H-吡咯-3-羧酸-1-基)氧杂环丁烷是由 3、4-二溴-2,2,5,5-四甲基-2,5-二氢-1H-吡咯-1-基氧基丹酰或 (2,2,5,5-四甲基-2,5-二氢-1H-吡咯-3-羧酸-1-基)氧丹酰为起始原料的反应的关键中间体。这种关键化合物可转化为叠氮特异性施陶丁格连接诱导自旋标签、氨基和硫醇特异性自旋标签或类似 MITO-CP 的抗增殖剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


