Photochemistry of (n-Bu4N)2[Pt(NO3)6] in acetonitrile.
IF 2.7
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Photochemical & Photobiological Sciences
Pub Date : 2024-04-01
Epub Date: 2024-03-02
DOI:10.1007/s43630-024-00550-5
引用次数: 0
Abstract
Photochemistry of the (n-Bu4N)2[Pt(NO3)6] complex in acetonitrile was studied by means of stationary photolysis and nanosecond laser flash photolysis. The primary photochemical process was found to be an intramolecular electron transfer followed by an escape of an •NO3 radical to the solution bulk. The spectra of two successive Pt(III) intermediates were detected in the microsecond time domain, and their spectral and kinetic characteristics were determined. These intermediates were identified as PtIII(NO3)52- and PtIII(NO3)4- complexes. Disproportionation of Pt(III) species resulted in formation of final Pt(II) products.
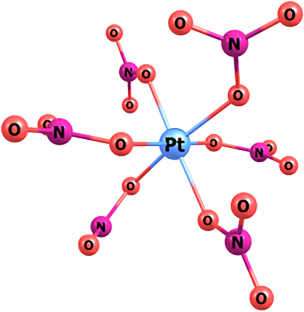
(n-Bu4N)2[Pt(NO3)6] 在乙腈中的光化学作用。
通过固定光解法和纳秒激光闪烁光解法研究了乙腈中 (n-Bu4N)2[Pt(NO3)6] 复合物的光化学过程。研究发现,主要的光化学过程是分子内电子转移,然后-NO3 自由基逸出到溶液中。在微秒时域中检测到了两个连续的 Pt(III)中间产物的光谱,并确定了它们的光谱和动力学特征。这些中间产物被确定为 PtIII(NO3)52- 和 PtIII(NO3)4- 复合物。铂(III)物种的歧化导致形成最终的铂(II)产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Photochemical & Photobiological Sciences
生物-生化与分子生物学
CiteScore
5.60
自引率
6.50%
发文量
201
审稿时长
2.3 months
期刊介绍:
A society-owned journal publishing high quality research on all aspects of photochemistry and photobiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


