Cryo-electron microscopy for GPCR research and drug discovery in endocrinology and metabolism
IF 31
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
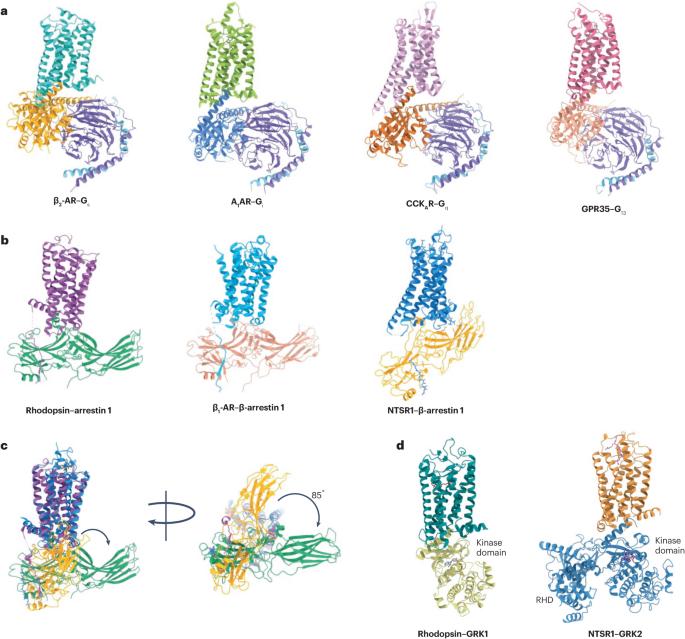
G protein-coupled receptors (GPCRs) are the largest family of cell surface receptors, with many GPCRs having crucial roles in endocrinology and metabolism. Cryogenic electron microscopy (cryo-EM) has revolutionized the field of structural biology, particularly regarding GPCRs, over the past decade. Since the first pair of GPCR structures resolved by cryo-EM were published in 2017, the number of GPCR structures resolved by cryo-EM has surpassed the number resolved by X-ray crystallography by 30%, reaching >650, and the number has doubled every ~0.63 years for the past 6 years. At this pace, it is predicted that the structure of 90% of all human GPCRs will be completed within the next 5–7 years. This Review highlights the general structural features and principles that guide GPCR ligand recognition, receptor activation, G protein coupling, arrestin recruitment and regulation by GPCR kinases. The Review also highlights the diversity of GPCR allosteric binding sites and how allosteric ligands could dictate biased signalling that is selective for a G protein pathway or an arrestin pathway. Finally, the authors use the examples of glycoprotein hormone receptors and glucagon-like peptide 1 receptor to illustrate the effect of cryo-EM on understanding GPCR biology in endocrinology and metabolism, as well as on GPCR-related endocrine diseases and drug discovery. This Review highlights how cryo-electron microscopy has revolutionized our understanding of G protein-coupled receptor (GPCR) functions. Specific examples are outlined that provide insights into GPCR biology and drug discovery in endocrinology and metabolism.
用于内分泌学和新陈代谢中 GPCR 研究和药物发现的冷冻电子显微镜。
G 蛋白偶联受体(GPCRs)是细胞表面受体中最大的家族,许多 GPCRs 在内分泌学和新陈代谢中起着至关重要的作用。低温电子显微镜(cryo-EM)在过去十年中彻底改变了结构生物学领域,尤其是 GPCR。自 2017 年发表第一对低温电子显微镜解析的 GPCR 结构以来,低温电子显微镜解析的 GPCR 结构数量已超过 X 射线晶体学解析的数量 30%,达到 650 个以上,并且在过去 6 年中每隔约 0.63 年翻一番。按照这一速度,预计在未来 5-7 年内将完成 90% 的人类 GPCR 结构研究。本综述强调了指导 GPCR 配体识别、受体激活、G 蛋白偶联、捕获素招募和 GPCR 激酶调控的一般结构特征和原理。该综述还强调了 GPCR 异构结合位点的多样性,以及异构配体如何决定对 G 蛋白通路或捕获素通路具有选择性的偏向信号。最后,作者以糖蛋白激素受体和胰高血糖素样肽 1 受体为例,说明了低温电子显微镜对理解内分泌学和新陈代谢中的 GPCR 生物学以及与 GPCR 相关的内分泌疾病和药物发现的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Endocrinology
医学-内分泌学与代谢
CiteScore
42.00
自引率
0.70%
发文量
158
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Endocrinology aspires to be the foremost platform for reviews and commentaries catering to the scientific communities it serves. The journal aims to publish articles characterized by authority, accessibility, and clarity, enhanced with easily understandable figures, tables, and other visual aids. The goal is to offer an unparalleled service to authors, referees, and readers, striving to maximize the usefulness and impact of each article. Nature Reviews Endocrinology publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians in the fields of endocrinology and metabolism. Its broad scope ensures that the work it publishes reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


