SERPINE2 promotes liver cancer metastasis by inhibiting c-Cbl-mediated EGFR ubiquitination and degradation
Abstract
Background
Liver cancer is a malignancy with high morbidity and mortality rates. Serpin family E member 2 (SERPINE2) has been reported to play a key role in the metastasis of many tumors. In this study, we aimed to investigate the potential mechanism of SERPINE2 in liver cancer metastasis.
Methods
The Cancer Genome Atlas database (TCGA), including DNA methylation and transcriptome sequencing data, was utilized to identify the crucial oncogene associated with DNA methylation and cancer progression in liver cancer. Data from the TCGA and RNA sequencing for 94 pairs of liver cancer tissues were used to explore the correlation between SERPINE2 expression and clinical parameters of patients. DNA methylation sequencing was used to detect the DNA methylation levels in liver cancer tissues and cells. RNA sequencing, cytokine assays, immunoprecipitation (IP) and mass spectrometry (MS) assays, protein stability assays, and ubiquitination assays were performed to explore the regulatory mechanism of SERPINE2 in liver cancer metastasis. Patient-derived xenografts and tumor organoid models were established to determine the role of SERPINE2 in the treatment of liver cancer using sorafenib.
Results
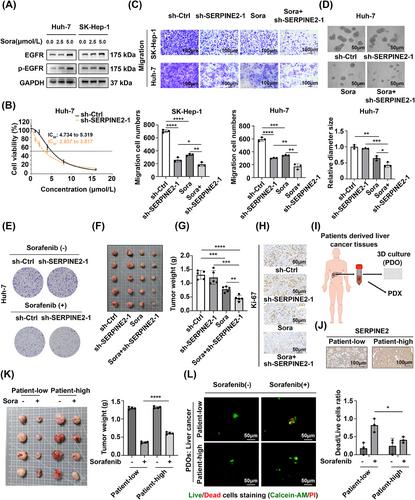
Based on the public database screening, SERPINE2 was identified as a tumor promoter regulated by DNA methylation. SERPINE2 expression was significantly higher in liver cancer tissues and was associated with the dismal prognosis in patients with liver cancer. SERPINE2 promoted liver cancer metastasis by enhancing cell pseudopodia formation, cell adhesion, cancer-associated fibroblast activation, extracellular matrix remodeling, and angiogenesis. IP/MS assays confirmed that SERPINE2 activated epidermal growth factor receptor (EGFR) and its downstream signaling pathways by interacting with EGFR. Mechanistically, SERPINE2 inhibited EGFR ubiquitination and maintained its protein stability by competing with the E3 ubiquitin ligase, c-Cbl. Additionally, EGFR was activated in liver cancer cells after sorafenib treatment, and SERPINE2 knockdown-induced EGFR downregulation significantly enhanced the therapeutic efficacy of sorafenib against liver cancer. Furthermore, we found that SERPINE2 knockdown also had a sensitizing effect on lenvatinib treatment.
Conclusions
SERPINE2 promoted liver cancer metastasis by preventing EGFR degradation via c-Cbl-mediated ubiquitination, suggesting that inhibition of the SERPINE2-EGFR axis may be a potential target for liver cancer treatment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


