Lamin A K97E leads to NF-κB-mediated dysfunction of inflammatory responses in dilated cardiomyopathy
Abstract
Background Information
Lamins are type V intermediate filament proteins underlying the inner nuclear membrane which provide structural rigidity to the nucleus, tether the chromosomes, maintain nuclear homeostasis, and remain dynamically associated with developmentally regulated regions of the genome. A large number of mutations particularly in the LMNA gene encoding lamin A/C results in a wide array of human diseases, collectively termed as laminopathies. Dilated Cardiomyopathy (DCM) is one such laminopathic cardiovascular disease which is associated with systolic dysfunction of left or both ventricles leading to cardiac arrhythmia which ultimately culminates into myocardial infarction.
Results
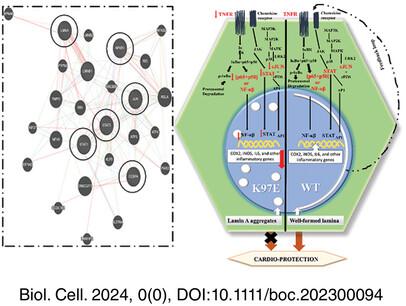
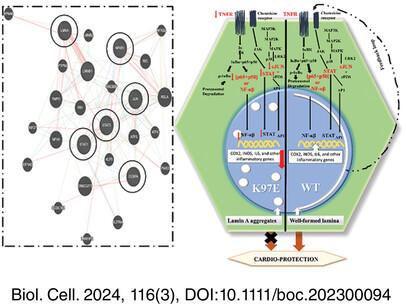
In this work, we have unraveled the epigenetic landscape to address the regulation of gene expression in mouse myoblast cell line in the context of the missense mutation LMNA 289A<G (Lys97Glu) that is found in DCM-afflicted patient with severe symptoms. Significant changes in H3-specific epigenetic modifications indicated a dysregulation in transcription machinery which was investigated by RNA sequencing analysis. The major pathways involved in IL-17 signaling, cellular response to interferon-beta and gamma, cytokine production, and related pathways are found to be downregulated. Analysis of the promoter sequences of the genes in the abovementioned pathways led us to the master regulator NF-κB and its regulatory network.
Conclusions
We report here for the first time that there is a significant downregulation of the NF-κB pathway, which has been implicated in cardio-protection elsewhere.
Significance
This provides a new pathophysiological explanation that correlates an LMNA mutation and dilated cardiomyopathy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


