Nucleosome-bound NR5A2 structure reveals pioneer factor mechanism by DNA minor groove anchor competition
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
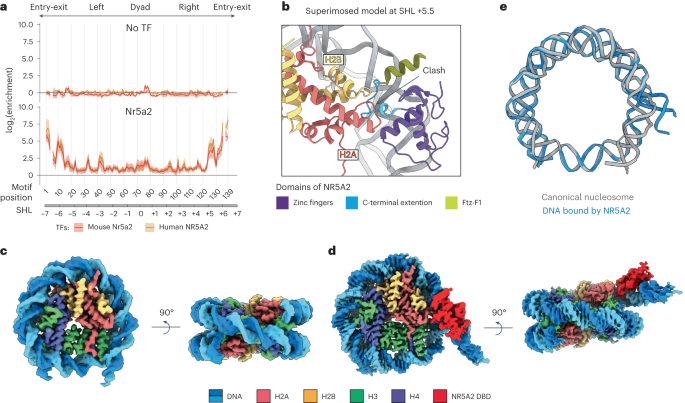
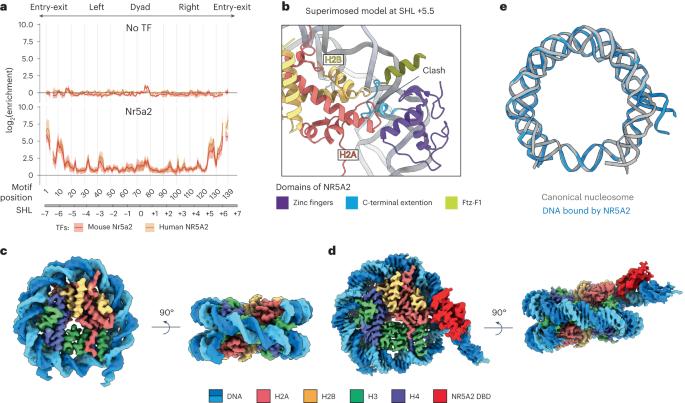
Gene expression during natural and induced reprogramming is controlled by pioneer transcription factors that initiate transcription from closed chromatin. Nr5a2 is a key pioneer factor that regulates zygotic genome activation in totipotent embryos, pluripotency in embryonic stem cells and metabolism in adult tissues, but the mechanism of its pioneer activity remains poorly understood. Here, we present a cryo-electron microscopy structure of human NR5A2 bound to a nucleosome. The structure shows that the conserved carboxy-terminal extension (CTE) loop of the NR5A2 DNA-binding domain competes with a DNA minor groove anchor of the nucleosome and releases entry-exit site DNA. Mutational analysis showed that NR5A2 D159 of the CTE is dispensable for DNA binding but required for stable nucleosome association and persistent DNA ‘unwrapping’. These findings suggest that NR5A2 belongs to an emerging class of pioneer factors that can use DNA minor groove anchor competition to destabilize nucleosomes and facilitate gene expression during reprogramming. The authors determined a cryo-EM structure of the pioneer factor NR5A2 bound to a nucleosome. NR5A2 releases nucleosomal DNA from histones by DNA minor anchor groove competition, providing a mechanism for pioneer factor activity during reprogramming
与核糖体结合的 NR5A2 结构揭示了 DNA 小沟锚竞争的先驱因子机制
自然和诱导重编程过程中的基因表达受先驱转录因子控制,这些因子从封闭的染色质中启动转录。Nr5a2是一种关键的先驱因子,它调控全能胚胎中的合子基因组激活、胚胎干细胞中的多能性以及成体组织中的新陈代谢,但人们对其先驱活动的机制仍然知之甚少。在这里,我们展示了与核小体结合的人类 NR5A2 的冷冻电镜结构。该结构显示,NR5A2 DNA 结合结构域的保守羧基末端延伸(CTE)环与核小体的 DNA 小沟锚竞争,并释放出入位点 DNA。突变分析表明,NR5A2 CTE 的 D159 对 DNA 结合是不可或缺的,但对稳定的核小体结合和持续的 DNA "解包裹 "却是必需的。这些研究结果表明,NR5A2 属于一类新兴的先驱因子,它可以利用 DNA 小沟锚竞争来破坏核小体的稳定性,并在重编程过程中促进基因表达。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


