Photothermal Microneedle Hydrogel Patch for Refractory Soft Tissue Injuries through Thermosensitized Anti-Inflammaging Modulation
引用次数: 0
Abstract
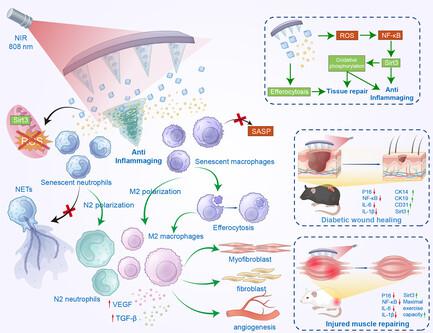
Soft tissue injuries (STIs) are the most common cause of extremity pain and motion dysfunction. Persistent inflammatory activation of immune cells characterized by senescence-associated secretory phenotype (SASP) and mitochondrial stress are considered the primary causes of STIs, a pathological process also termed inflammaging. Meanwhile, scavenging excessive “cellular waste” in the inflammaging microenvironment and further activating tissue repair processes remain elusive. Herein, an anti-inflammaging photothermal hydrogel microneedle patch for treating STIs is developed. Taurine-loaded Prussian blue nanoparticles (Taurine@PB) are encapsulated in a methacrylate-based hyaluronic acid hydrogel (HAMA) and further fabricated into taurine@PB@HAMA@microneedles (TPH@MN) patches. The acidic microenvironment of chronic inflammation and mild photothermal effects promote taurine release and anti-inflammaging immunomodulation, inhibiting mitochondrial stress via the SIRT3-NF-κB axis to promote glycolytic metabolic microenvironment of neutrophils reprogramming toward oxidative phosphorylation metabolism. Furthermore, TPH@MN activates macrophage efferocytosis and initiates the process of tissue repair. In mouse models of chronic diabetic wounds and tibialis anterior (TA) muscle injury, TPH@MN inhibits SASP expression and promotes STIs healing through thermosensitized anti-inflammaging immunomodulation. In summary, TPH@MN circumvents the side effects of systemic administration, providing new translatable options in the treatment modalities for patients suffering from STIs worldwide.
通过热敏抗炎调节治疗难治性软组织损伤的光热微针水凝胶贴片
软组织损伤(STI)是导致四肢疼痛和运动功能障碍的最常见原因。以衰老相关分泌表型(SASP)和线粒体应激为特征的免疫细胞的持续炎症激活被认为是导致软组织损伤的主要原因,这一病理过程也被称为炎症老化。与此同时,如何清除炎症微环境中过多的 "细胞废物 "并进一步激活组织修复过程仍是一个难题。在此,我们开发了一种用于治疗性传播感染的抗炎光热水凝胶微针贴片。牛磺酸负载的普鲁士蓝纳米粒子(Taurine@PB)被封装在甲基丙烯酸酯基透明质酸水凝胶(HAMA)中,并进一步制成牛磺酸@PB@HAMA@微针(TPH@MN)贴片。慢性炎症的酸性微环境和温和的光热效应促进了牛磺酸的释放和抗炎免疫调节,通过 SIRT3-NF-κB 轴抑制线粒体应激,从而促进中性粒细胞的糖酵解代谢微环境向氧化磷酸化代谢重编程。此外,TPH@MN 还能激活巨噬细胞的排泄,启动组织修复过程。在慢性糖尿病伤口和胫骨前肌(TA)损伤的小鼠模型中,TPH@MN 可抑制 SASP 的表达,并通过热敏抗炎免疫调节促进 STI 的愈合。总之,TPH@MN 避免了全身用药的副作用,为全球性传播感染患者的治疗模式提供了新的可转化选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
文献相关原料
公司名称
产品信息
阿拉丁
Potassium ferricyanide (K3[Fe(CN)6])
阿拉丁
taurine
阿拉丁
PVP
阿拉丁
MB

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


