Impact of different sequential triple oral combination therapies based selexipag on outcomes in pulmonary arterial hypertension
Abstract
Background
While the GRIPHON study and others have confirmed the efficacy and safety of selexipag with single, dual, and initial triple combination therapy for patients with pulmonary arterial hypertension (PAH), multicenters studies concerning diverse triple oral combination therapies based on selexipag are limited.
Hypothesis
This study was conducted to evaluate the effects of various sequential triple oral combination therapies on PAH outcomes.
Methods
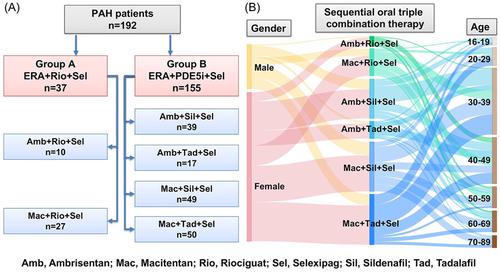
A retrospective study was carried out involving 192 patients from 10 centers, who were receiving sequential triple oral combination therapy consisting of an endothelin receptor antagonist (ERA), a phosphodiesterase 5 inhibitor (PDE5i)/riociguat and selexipag. Clinical parameters, event-free survival, and all-cause survival were assessed and analyzed at baseline and posttreatment.
Results
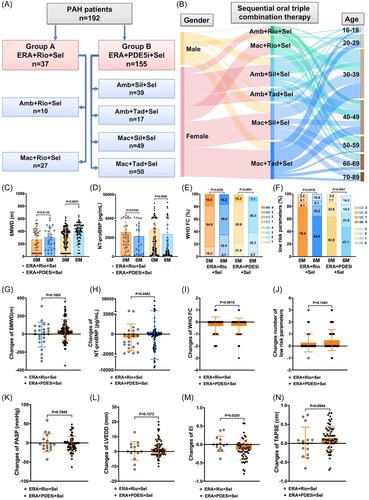
Among the 192 patients, 37 were treated with ERA + riociguat + selexipag, and 155 patients received ERA + PDE5i + selexipag. Both sequential triple oral combination therapies improved the World Health Organization functional class and raised the count of low-risk parameters. As a result of the larger patients' population in the ERA + PDE5i + selexipag group, these individuals exhibited significant increases in 6-minute walking distance (6MWD), pulmonary arterial systolic pressure, pulmonary arterial pressure, right ventricle, and eccentricity index, and significant decreases in N-terminal probrain natriuretic peptide after 6 months of treatment. Nevertheless, both sequential triple oral combination therapy groups demonstrated similar shifts in these clinical parameters between baseline and 6 months. Baseline 6MWD and mean pulmonary arterial pressure were independent predictors of survival in patients undergoing ERA + PDE5i + selexipag therapy. Importantly, no significant differences were found in 6-month event-free survival and all-cause survival between two groups.
Conclusions
Different oral sequential triple combination therapies based on selexipag could comparably improve outcomes in patients with PAH.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


