Selenium chemistry for spatio-selective peptide and protein functionalization
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The ability to construct a peptide or protein in a spatio-specific manner is of great interest for therapeutic and biochemical research. However, the various functional groups present in peptide sequences and the need to perform chemistry under mild and aqueous conditions make selective protein functionalization one of the greatest synthetic challenges. The fascinating paradox of selenium (Se) — being found in both toxic compounds and also harnessed by nature for essential biochemical processes — has inspired the recent exploration of selenium chemistry for site-selective functionalization of peptides and proteins. In this Review, we discuss such approaches, including metal-free and metal-catalysed transformations, as well as traceless chemical modifications. We report their advantages, limitations and applications, as well as future research avenues. The unique properties of selenium have been exploited in protein science. This Review highlights the recent applications of selenium chemistry in protein chemical synthesis, modification, folding, stabilization, the preparation of therapeutic proteins and more.
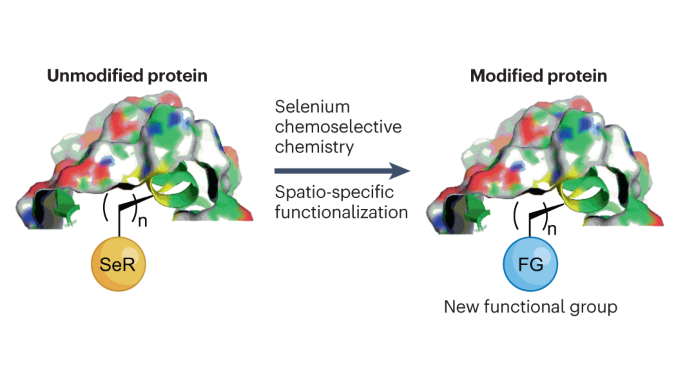
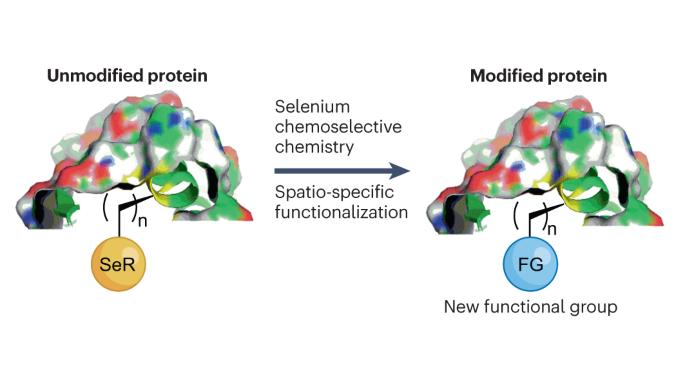
用于多肽和蛋白质空间选择功能化的硒化学。
以空间特异性方式构建多肽或蛋白质的能力对治疗和生化研究具有重大意义。然而,肽序列中存在的各种官能团以及在温和的水性条件下进行化学反应的需要,使得选择性蛋白质官能化成为最大的合成挑战之一。硒(Se)既存在于有毒化合物中,又被自然界用于重要的生化过程,这一令人着迷的悖论激发了人们最近对硒化学的探索,以实现多肽和蛋白质的位点选择性官能化。在本综述中,我们将讨论这些方法,包括无金属和金属催化转化以及无痕化学修饰。我们报告了这些方法的优势、局限性和应用,以及未来的研究方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


