Gut microbial metabolite trimethylamine N-oxide induces aortic dissection
Abstract
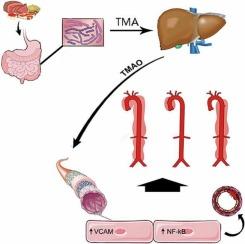
Aortic dissection (AD) is the most catastrophic vascular disease with a high mortality rate. Trimethylamine N-oxide (TMAO), a gut microbial metabolite, has been implicated in the pathogenesis of cardiovascular diseases. However, the role of TMAO in AD and the underlying mechanisms remain unclear. This study aimed to explore the effects of TMAO on AD. Plasma and fecal samples from patients with AD and healthy individuals were collected to analyze TMAO levels and gut microbial species, respectively. The plasma levels of TMAO were significantly higher in 253 AD patients compared with those in 98 healthy subjects (3.47, interquartile range (IQR): 2.33 to 5.18 μM vs. 1.85, IQR: 1.40 to 3.35 μM; p < 0.001). High plasma TMAO levels were positively associated with AD severity. An increase in the relative abundance of TMA-producing genera in patients with AD was revealed using 16S rRNA sequencing. In the angiotensin II or β-aminopropionitrile-induced rodent model of AD, mice fed a TMAO-supplemented diet were more likely to develop AD compared to mice fed a normal diet. Conversely, TMAO depletion mitigated AD formation in the BAPN model. RNA sequencing of aortic endothelial cells isolated from mice administered TMAO revealed significant upregulation of genes involved in inflammatory pathways. The in vitro experiments verified that TMAO promotes endothelial dysfunction and activates nuclear factor (NF)-κB signaling. The in vivo BAPN-induced AD model confirmed that TMAO increased aortic inflammation. Our study demonstrates that the gut microbial metabolite TMAO aggravates the development of AD at least in part by inducing endothelial dysfunction and inflammation. This study provides new insights into the etiology of AD and ideas for its management.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


