Cryo-EM structure of human sphingomyelin synthase and its mechanistic implications for sphingomyelin synthesis
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
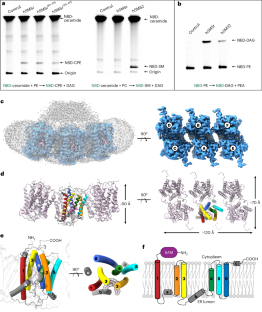
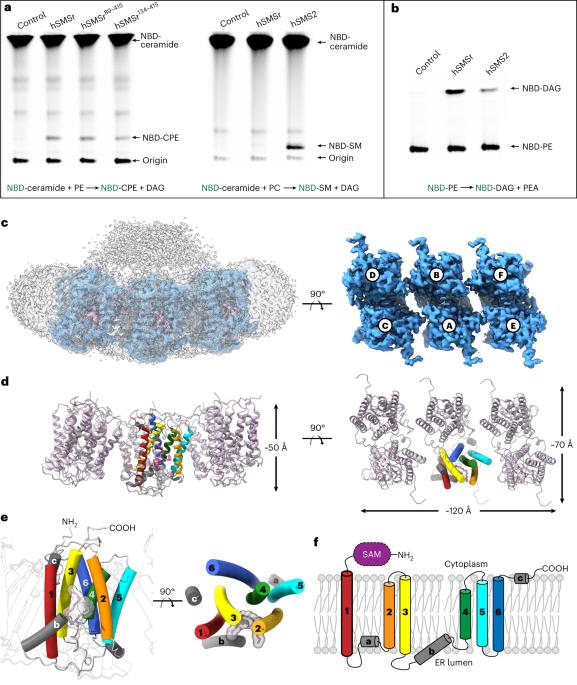
Sphingomyelin (SM) has key roles in modulating mammalian membrane properties and serves as an important pool for bioactive molecules. SM biosynthesis is mediated by the sphingomyelin synthase (SMS) family, comprising SMS1, SMS2 and SMS-related (SMSr) members. Although SMS1 and SMS2 exhibit SMS activity, SMSr possesses ceramide phosphoethanolamine synthase activity. Here we determined the cryo-electron microscopic structures of human SMSr in complexes with ceramide, diacylglycerol/phosphoethanolamine and ceramide/phosphoethanolamine (CPE). The structures revealed a hexameric arrangement with a reaction chamber located between the transmembrane helices. Within this structure, a catalytic pentad E–H/D–H–D was identified, situated at the interface between the lipophilic and hydrophilic segments of the reaction chamber. Additionally, the study unveiled the two-step synthesis process catalyzed by SMSr, involving PE–PLC (phosphatidylethanolamine–phospholipase C) hydrolysis and the subsequent transfer of the phosphoethanolamine moiety to ceramide. This research provides insights into the catalytic mechanism of SMSr and expands our understanding of sphingolipid metabolism. Researchers unveiled the structural details of sphingomyelin synthase (SMSr), shedding light on its role in sphingolipid biosynthesis. SMSr transfers the phosphoethanolamine from PE to ceramide, adding complexity to the field of lipid homeostasis.
人类鞘磷脂合成酶的低温电子显微镜结构及其对鞘磷脂合成的机理影响
鞘磷脂(SM)在调节哺乳动物膜特性方面起着关键作用,是生物活性分子的重要来源。鞘磷脂的生物合成由鞘磷脂合成酶(SMS)家族介导,包括 SMS1、SMS2 和 SMS 相关(SMSr)成员。虽然 SMS1 和 SMS2 具有 SMS 活性,但 SMSr 具有神经酰胺磷乙醇胺合酶活性。在这里,我们测定了人类 SMSr 与神经酰胺、二酰甘油/磷脂酰乙醇胺和神经酰胺/磷脂酰乙醇胺(CPE)复合物的冷冻电镜结构。这些结构显示,SMSr 呈六聚体排列,反应室位于跨膜螺旋之间。在该结构中,发现了位于反应室亲脂段和亲水段界面上的催化五元体 E-H/D-H-D。此外,研究还揭示了 SMSr 催化的两步合成过程,包括 PE-PLC(磷脂酰乙醇胺-磷脂酶 C)水解以及随后磷脂酰乙醇胺分子向神经酰胺的转移。这项研究深入揭示了 SMSr 的催化机理,拓展了我们对鞘脂代谢的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


