Melt adsorption of poly(tert-butyl methacrylate) and poly(ethyl methacrylate) on silica studied with chip nanocalorimetry
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
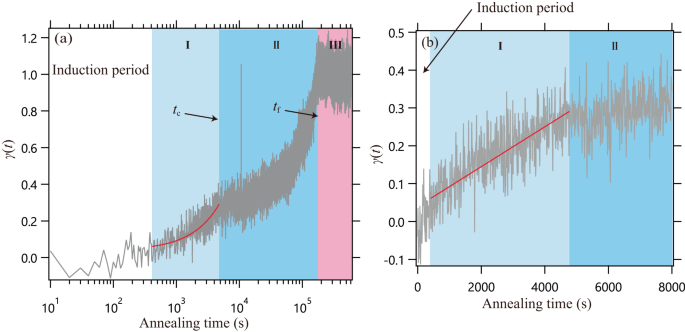
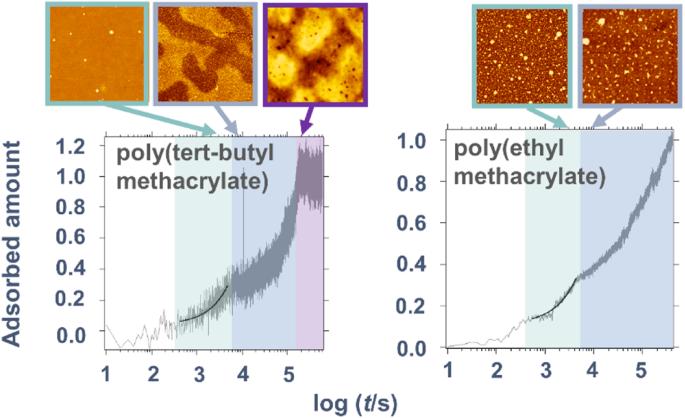
Irreversible adsorption of polymer chains from a melt on a substrate surface can be strongly affected by interfacial interactions. In this study, we examined the adsorption of two polymers, poly(tert-butyl methacrylate) (PtBMA) and poly(ethyl methacrylate) (PEMA), on a silica surface at temperatures above their glass transition temperatures. The degree of adsorption γ(t) over time was evaluated with variations of storage heat capacity determined with alternating current chip nanocalorimetry (in-situ measurement of a buried interface). γ(t) revealed two-step profiles for both polymers. At the second stage of adsorption (regime II), the slope of a plot of γ(t) vs. log t increased as adsorption proceeded; this trend has not been reported for other polymers and may be characteristic of the present polymers. The trend observed in regime II suggested that the unadsorbed free chains near the interface became less mobile and were incorporated into the adsorbed layer via interactions with the tails of the chains directly attached to the substrate surface. The increasing slope in regime II was more prominent for PtBMA than for PEMA. In addition, a difference was observed for PtBMA and PEMA in the atomic force microscopy images of the exposed adsorption layer surfaces. We examined the adsorption behavior of poly(tert-butyl methacrylate) (PtBMA) and poly(ethyl methacrylate) (PEMA), on a silica surface. Time-evolution of the degree of adsorption γ(t) was evaluated with chip nanocalorimetry. γ(t) revealed a two-step profile for both polymers. At the second stage of adsorption, the slope of γ(t) vs. log t increased as adsorption proceeded; this trend has not been reported for other polymers so far. In addition, atomic force microscopy images of the adsorbed layers revealed corresponding evolutions of the morphologies.
用芯片纳米焦度计研究聚甲基丙烯酸叔丁酯和聚甲基丙烯酸乙酯在二氧化硅上的熔融吸附作用
熔体中的聚合物链在基底表面的不可逆吸附会受到界面相互作用的强烈影响。在本研究中,我们研究了聚甲基丙烯酸叔丁酯(PtBMA)和聚甲基丙烯酸乙酯(PEMA)这两种聚合物在高于其玻璃化转变温度时在二氧化硅表面的吸附情况。吸附程度γ(t)随时间的变化通过交变电流芯片纳米焦度计(埋藏界面的原位测量)测定的存储热容量变化进行评估。两种聚合物的γ(t)都显示出两个阶段的曲线。在吸附的第二阶段(阶段 II),随着吸附的进行,γ(t) 对 log t 曲线的斜率增加;这种趋势在其他聚合物中没有报道过,可能是本聚合物的特征。在状态 II 中观察到的趋势表明,界面附近未吸附的游离链的流动性变小,并通过与直接附着在基底表面的链尾的相互作用而被吸附到吸附层中。与 PEMA 相比,PtBMA 在第二阶段的斜率增加更为明显。此外,在暴露的吸附层表面的原子力显微镜图像中也观察到了 PtBMA 和 PEMA 的差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


