Computational demystification of iron carbonyls formation under syngas environment
IF 6.6
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
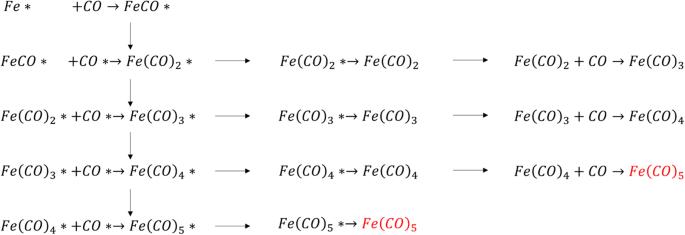
Iron pentacarbonyl (IPC) gas forms upon the reaction of carbon monoxide with Fe containing metallic surfaces under gas reforming conditions. IPC formation can sometimes reach alarming levels that cause metal loss, pipeline thinning corrosion, catalyst poisoning, and contamination of sensitive industrial equipment. In this work, we demystify using multiscale computational modeling the mechanism of Iron pentacarbonyl formation: Density functional theory (DFT) is used to explore various catalytic reactions that involve a Fe adatom reacting with adsorbed carbon monoxide. Our calculated carbonyls desorption barriers on a perfect and clean Fe surface are too high to allow the carbonyls to form then desorb at temperatures <500 K at the rates reported experimentally. Most importantly, our calculations indicate that a high CO surface coverage, in addition to the presence of Fe adatoms, favors carbonyl formation and its desorption towards the flowing gas medium. Using insights extracted from ab initio molecular dynamics simulations, we propose that the most plausible IPC formation mechanism consists of: (1) on surface reactions of adsorbed CO molecules with an Fe adatom to form iron tricarbonyl (Fe(CO)3*) molecules; (2) an adsorbate assisted movement of iron tricarbonyl on top of the CO adlayer; and (3) the interaction of iron tricarbonyl with CO molecules from the gaseous medium eventually leading to iron adatom removal as Fe(CO)5 gas.
合成气环境下羰基铁形成的计算解密
在气体重整条件下,一氧化碳与含铁金属表面发生反应后会形成五羰基铁(IPC)气体。五羰基铁的形成有时会达到惊人的程度,导致金属损失、管道腐蚀变细、催化剂中毒以及敏感工业设备污染。在这项工作中,我们利用多尺度计算模型揭开了五羰基铁形成机理的神秘面纱:密度泛函理论(DFT)用于探索涉及铁原子与吸附的一氧化碳反应的各种催化反应。我们计算出的羰基在完美洁净的铁表面上的解吸障碍过高,以至于羰基无法在 500 K 的温度下以实验报告的速率形成和解吸。最重要的是,我们的计算表明,除了铁原子的存在之外,高 CO 表面覆盖率也有利于羰基的形成及其在流动气体介质中的解吸。利用从 ab initio 分子动力学模拟中提取的观点,我们提出最合理的 IPC 形成机制包括(1) 吸附的一氧化碳分子与铁原子发生表面反应,形成三羰基铁(Fe(CO)3*)分子;(2) 三羰基铁在一氧化碳吸附层顶部的吸附剂辅助运动;以及 (3) 三羰基铁与气体介质中的一氧化碳分子相互作用,最终导致铁原子以 Fe(CO)5 气体的形式脱附。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

npj Materials Degradation
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
7.80
自引率
7.80%
发文量
86
审稿时长
6 weeks
期刊介绍:
npj Materials Degradation considers basic and applied research that explores all aspects of the degradation of metallic and non-metallic materials. The journal broadly defines ‘materials degradation’ as a reduction in the ability of a material to perform its task in-service as a result of environmental exposure.
The journal covers a broad range of topics including but not limited to:
-Degradation of metals, glasses, minerals, polymers, ceramics, cements and composites in natural and engineered environments, as a result of various stimuli
-Computational and experimental studies of degradation mechanisms and kinetics
-Characterization of degradation by traditional and emerging techniques
-New approaches and technologies for enhancing resistance to degradation
-Inspection and monitoring techniques for materials in-service, such as sensing technologies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


