Repeat patient testing-quality control compared to commercial quality control material for the Sysmex XT-2000iV hematology analyzer in a multi-site veterinary laboratory
Abstract
Background
Quality control material (QCM) for hematology in veterinary laboratories is limited, and repeat patient testing quality control (RPT-QC) is an alternative method using excess matrix-specific samples.
Objectives
This study aimed to determine if median differences between RPT-QC analyses for each time interval for RBC, HGB, HCT, and WBC were the same, determine if unified RPT-QC limits can be applied to a network of veterinary laboratories, compare the performance of RPT-QC to commercial QCM for the reference analyzer and evaluate the experience over a 4 month period and design, improve and implement an automated spreadsheet for RPT-QC data management.
Methods
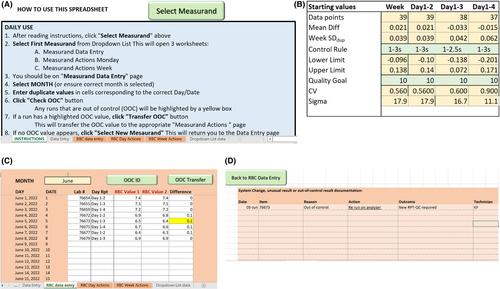
The potential to unify individual analyzer RPT-QC limits for red blood cells (RBC), hematocrit (HCT), hemoglobin (HGB), and white blood cells (WBC) on multi-site Sysmex XT-2000-iV analyzers was explored by a difference of means test and confidence interval determination for the median difference for each network analyzer in comparison to the network reference analyzer. User experience of an automated RPT-QC data management Excel spreadsheet was collected by user feedback during monthly meetings. Numbers of out-of-control results and the root causes for these for RPT-QC were compared against those of a commercial QCM over a 4-month period.
Results
Differences between individual analyzer RPT-QC limits were too large to allow for unification of network limits. The automated spreadsheet successfully highlighted out-of-control events for RPT-QC. Trends or shifts were more frequent for commercial QCM based on observed performance and a 1–2.5 s QC rule than for RPT-QC. Following routine troubleshooting, RPT-QC out-of-control events were resolved with an alternative RPT-QC sample indicating random error associated with excessive deterioration. Use of an automated spreadsheet for recording RPT-QC, documentation and troubleshooting of out-of-control events, and collating monthly summary calculations were considered an asset in laboratory quality management.
Conclusions
RPT-QC can be successfully implemented and integrated into a multi-site veterinary laboratory. Individual analyzer RPT-QC limit generation is recommended. The deterioration of commercial QCM caused shifts or trends in QC results, which initiated more repeat analyses and investigations than did RPT-QC.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


