Beyond genetics: driving cancer with the tumour microenvironment behind the wheel
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
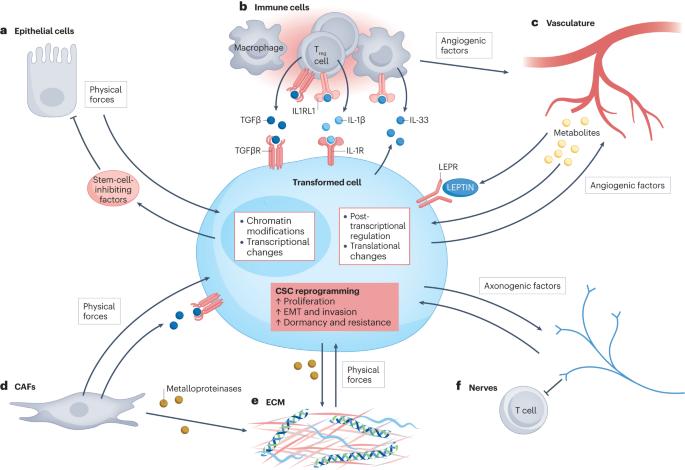
Cancer has long been viewed as a genetic disease of cumulative mutations. This notion is fuelled by studies showing that ageing tissues are often riddled with clones of complex oncogenic backgrounds coexisting in seeming harmony with their normal tissue counterparts. Equally puzzling, however, is how cancer cells harbouring high mutational burden contribute to normal, tumour-free mice when allowed to develop within the confines of healthy embryos. Conversely, recent evidence suggests that adult tissue cells expressing only one or a few oncogenes can, in some contexts, generate tumours exhibiting many of the features of a malignant, invasive cancer. These disparate observations are difficult to reconcile without invoking environmental cues triggering epigenetic changes that can either dampen or drive malignant transformation. In this Review, we focus on how certain oncogenes can launch a two-way dialogue of miscommunication between a stem cell and its environment that can rewire downstream events non-genetically and skew the morphogenetic course of the tissue. We review the cells and molecules of and the physical forces acting in the resulting tumour microenvironments that can profoundly affect the behaviours of transformed cells. Finally, we discuss possible explanations for the remarkable diversity in the relative importance of mutational burden versus tumour microenvironment and its clinical relevance. In their Review article, Fuchs and colleagues discuss how a single or a few mutations in adult cells can lead to invasive cancers without a high mutational burden, demonstrating that non-genetic factors induce the epigenetic changes necessary for tumorigenesis.
超越遗传学:以肿瘤微环境驱动癌症发展。
长期以来,癌症一直被视为一种累积突变的遗传疾病。有研究表明,衰老组织中往往充斥着复杂的致癌基因克隆,它们与正常组织中的同类基因似乎和谐共存,这进一步证实了这一观点。然而,同样令人困惑的是,如果让携带高突变负荷的癌细胞在健康胚胎中发育,它们又是如何形成正常、无肿瘤的小鼠的?相反,最近的证据表明,只表达一种或几种癌基因的成体组织细胞在某些情况下也能产生肿瘤,表现出恶性侵袭性癌症的许多特征。如果不考虑引发表观遗传学变化的环境线索,就很难调和这些不同的观察结果。在这篇综述中,我们将重点讨论某些癌基因如何在干细胞与其环境之间启动双向交流对话,从而非遗传地重新连接下游事件,并歪曲组织的形态发生过程。我们回顾了由此产生的肿瘤微环境中的细胞、分子和物理力,它们会深刻影响转化细胞的行为。最后,我们将讨论突变负荷与肿瘤微环境的相对重要性存在显著差异的可能原因及其临床意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


