Tolerance-inducing therapies in coeliac disease — mechanisms, progress and future directions
IF 45.9
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Coeliac disease is an autoinflammatory condition caused by immune reactions to cereal gluten proteins. Currently, the only available treatment for the condition is a lifelong avoidance of gluten proteins in the diet. There is an unmet need for alternative therapies. Coeliac disease has a strong association with certain HLA−DQ allotypes (DQ2.5, DQ2.2 and DQ8), and these disease-associated HLA-DQ molecules present deamidated gluten peptides to gluten-specific CD4+ T cells. The gluten-specific CD4+ T cells are the drivers of the immune reactions leading to coeliac disease. Once established, the clonotypes of gluten-specific CD4+ T cells persist for decades, explaining why patients must adhere to a gluten-free diet for life. Given the key pathogenic role of gluten-specific CD4+ T cells, tolerance-inducing therapies that target these T cells are attractive for treatment of the disorder. Lessons learned from coeliac disease might provide clues for treatment of other HLA-associated diseases for which the disease-driving antigens are unknown. Thus, intensive efforts have been and are currently implemented to bring an effective tolerance-inducing therapy for coeliac disease. This Review discusses mechanisms of the various approaches taken, summarizing the progress made, and highlights future directions in this field. Coeliac disease is an autoinflammatory disease, with the only available treatment being a lifelong gluten-free diet. Alternative therapeutic approaches are needed. This Review explores the concept of tolerance-inducing therapies for coeliac disease, highlighting the underlying mechanisms, progress, challenges and future directions.
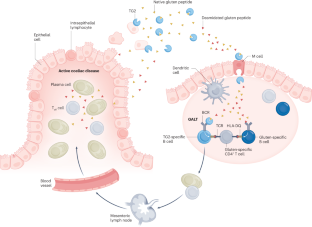
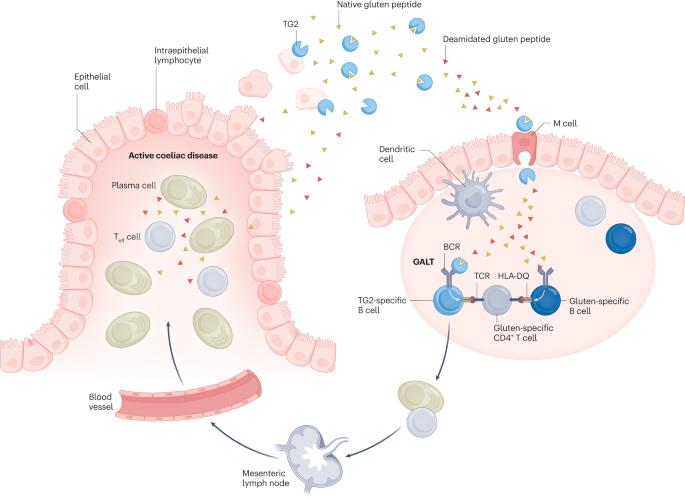
乳糜泻的耐受诱导疗法--机制、进展和未来方向
乳糜泻是一种由谷类麸质蛋白引起的免疫反应导致的自身炎症。目前,治疗这种疾病的唯一方法是终生避免在饮食中摄入麸质蛋白。替代疗法的需求尚未得到满足。乳糜泻与某些 HLA-DQ 异型(DQ2.5、DQ2.2 和 DQ8)密切相关,这些与疾病相关的 HLA-DQ 分子会向麸质特异性 CD4+ T 细胞展示脱氨麸质肽。麸质特异性 CD4+ T 细胞是导致乳糜泻的免疫反应的驱动力。麸质特异性 CD4+ T 细胞的克隆型一旦形成,就会持续数十年,这也解释了为什么患者必须终生坚持无麸质饮食。鉴于麸质特异性 CD4+ T 细胞的关键致病作用,针对这些 T 细胞的耐受诱导疗法对治疗这种疾病很有吸引力。从乳糜泻中汲取的经验教训可能会为治疗其他 HLA 相关疾病提供线索,因为这些疾病的致病抗原尚不清楚。因此,人们一直在努力为乳糜泻提供有效的耐受诱导疗法。本综述讨论了所采取的各种方法的机制,总结了所取得的进展,并强调了该领域未来的发展方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
52.30
自引率
0.60%
发文量
147
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Gastroenterology & Hepatology aims to serve as the leading resource for Reviews and commentaries within the scientific and medical communities it caters to. The journal strives to maintain authority, accessibility, and clarity in its published articles, which are complemented by easily understandable figures, tables, and other display items. Dedicated to providing exceptional service to authors, referees, and readers, the editorial team works diligently to maximize the usefulness and impact of each publication.
The journal encompasses a wide range of content types, including Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements, all pertinent to gastroenterologists and hepatologists. With its broad scope, Nature Reviews Gastroenterology & Hepatology ensures that its articles reach a diverse audience, aiming for the widest possible dissemination of valuable information.
Nature Reviews Gastroenterology & Hepatology is part of the Nature Reviews portfolio of journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


