Engineering effective separation of photo-assisted charge carriers by provoking fenton-like reaction for degradation of rhodamine B dye
Abstract
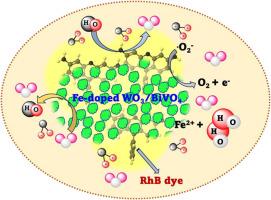
Developing a photocatalyst for environmental remediation with extortionate visible light absorption capability and low reunion of photogenerated charge carriers is of tremendous interest. Considering this, the present work reports the fabrication of a low-cost and eco-friendly Fe-doped WO3/BiVO4 photocatalyst prepared by the facile one-step hydrothermal technique. The photocatalyst outperformed in the removal of rhodamine B dye than the pristine samples. The XRD and Raman spectroscopy analysis affirms the successful doping of the Fe cations within the WO3 crystal structure. The absorption studies reveal the redshift to higher wavelengths which elucidates the enhancement of oxygen vacancy, and the band gap value changes are also apparent due to the heterojunction scheme of the photocatalyst. The time-resolved photoluminescence studies substantiate the effective reduction in the recombination rate with an average lifetime of 364 ns proving it to be an effective photocatalyst for the removal of rhodamine B dye. The catalyst revealed outstanding performance with 94.3 % removal of rhodamine B dye within 6 h. However, the removal efficiency was higher at pH 14 with a degradation of 92.6 % (100 min) corroborating that the influence of hydroxyl radical greatly facilitates a Fenton-like reaction that provokes the degradation process faster. It was further confirmed from the scavenging analysis that, with the addition of an H2O2 scavenger the degradation rate is fast due to the formation of hydroxyl radicals that emerged from the fusion of H2O2 with superoxide radicals. This outperformance validates the competency of the photocatalyst in the removal of organic pollutants.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


