Reagent- and solvent-controlled product divergence in the reaction of trifluorodiazoethane with arylidene-1,3-indanediones
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
An interesting product divergence in the reaction of trifluorodiazoethane with arylidene-1,3-indanediones is reported. The reaction conducted using 10 mol% of silver carbonate afforded trifluoromethylspiropyrazolines, while with excess of cesium fluoride in methanol, the cycloaddition was followed by nucleophilic ring opening of the spiropyrazoline to afford trifluoromethylated o-carbomethoxybenzoylpyrazolines. Both protocols work under mild reaction conditions and exhibit good functional group tolerance.
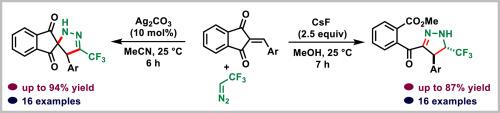
三氟二乙烷与芳基-1,3-茚二酮反应中由试剂和溶剂控制的产物差异
据报道,在三氟二氮杂环丁烷与芳基-1,3-茚二酮的反应中,出现了有趣的产物分化现象。使用 10 摩尔%的碳酸银进行的反应得到了三氟甲基螺吡唑啉,而在甲醇中加入过量的氟化铯,环化反应后螺吡唑啉亲核开环,得到了三氟甲基邻甲氧基苯甲酰吡唑啉。这两种方案都能在温和的反应条件下进行,并表现出良好的官能团耐受性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


