Preliminary X-ray Diffraction Analysis of Purine Nucleoside Phosphorylase from the Haloalkaliphilic Bacterium Halomonas chromatireducens
IF 0.6
4区 材料科学
Q4 CRYSTALLOGRAPHY
引用次数: 0
Abstract
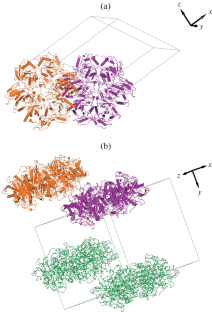
Crystals of the enzyme purine nucleoside phosphorylase from the extremophilic bacterium Halomonas Chromatireducens AGD 8-3, suitable for X-ray diffraction, were grown by the vapor-diffusion method. The X-ray diffraction data were collected from these crystals at the Belok beamline of the Kurchatov synchrotron radiation source (National Research Centre “Kurchatov Institute”) at 100 K to 1.80 Å resolution. The X-ray diffraction data were processed in the space groups P1, P2, P21, and P622. The structure was solved by the molecular replacement method taking into account the twinning in the space groups P21 and P1 with one and two hexamers of the enzyme per asymmetric unit, respectively.
卤代嗜碱细菌 Halomonas chromatireducens 的嘌呤核苷磷酸酶的初步 X 射线衍射分析
摘要通过蒸发扩散法从嗜极细菌 Halomonas Chromatireducens AGD 8-3 中生长出适合 X 射线衍射的嘌呤核苷磷酸化酶晶体。这些晶体的 X 射线衍射数据是在库尔恰托夫同步辐射源(国家研究中心 "库尔恰托夫研究所")的 Belok 光束线以 100 K 的分辨率 1.80 Å 采集的。X 射线衍射数据在空间群 P1、P2、P21 和 P622 中进行了处理。考虑到 P21 和 P1 空间群中的孪生结构,每个不对称单元分别有一个和两个酶的六聚体,因此采用分子置换法对该结构进行了求解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Crystallography Reports
化学-晶体学
CiteScore
1.10
自引率
28.60%
发文量
96
审稿时长
4-8 weeks
期刊介绍:
Crystallography Reports is a journal that publishes original articles short communications, and reviews on various aspects of crystallography: diffraction and scattering of X-rays, electrons, and neutrons, determination of crystal structure of inorganic and organic substances, including proteins and other biological substances; UV-VIS and IR spectroscopy; growth, imperfect structure and physical properties of crystals; thin films, liquid crystals, nanomaterials, partially disordered systems, and the methods of studies.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


