Hemostatic imbalance induced by tamoxifen in estrogen receptor-positive breast cancer patients: An observational study
Abstract
Background
Estrogen receptor (ER)-positive (ER+) breast cancer accounts for approximately 75% of all breast cancers. Tamoxifen, a selective estrogen receptor modulator, is the standard adjuvant treatment. Although better tolerated than aromatase inhibitors, tamoxifen increases the risk of venous thromboembolism (VTE) 1.4-fold.
Aim
To assess the hemostatic imbalance induced by tamoxifen in adjuvant treatment of ER+ breast cancer.
Method
Twenty-five patients in remission from ER+ breast cancer under tamoxifen were included. One hundred and thirty one age- and BMI-matched healthy controls were included to establish reference ranges of thrombin generation assay (TGA) parameters. TGA was performed in the absence and presence of exogenous activated protein C (APC) to calculate the normalized APC sensitivity ratio (nAPCsr), a marker of APC resistance.
Results
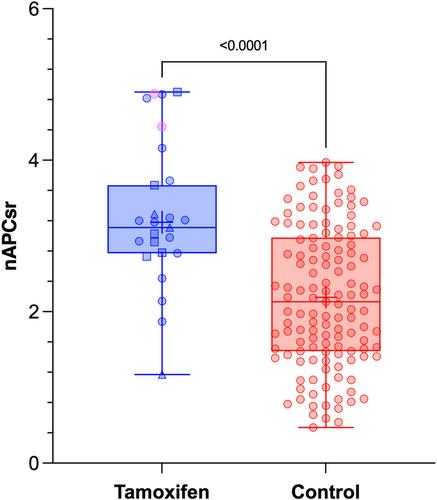
All TG parameters except the endogenous thrombin potential (ETP) (−APC) were significantly impacted by tamoxifen (p < 0.001). In absence of APC, regardless of TGA parameters, at least 50% of results were outside the reference ranges except for ETP, which was above the upper reference limit in only two individuals. The most impacted parameter was the Peak Height with 52% (−APC) and 80% (+APC) of results above the upper reference range limit, respectively. The nAPCsr was significantly higher in tamoxifen users (mean ± standard deviation = 3.18 ± 0.91) compared to the control group (2.19 ± 0.92, p < 0.0001).
Conclusion
This observational study showed that patients in remission from ER+ breast cancer taking tamoxifen had altered thrombin generation, as well as an acquired APC resistance. Moreover, this is the first study using the validated ETP-based APC resistance assay in tamoxifen-treated patients.
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


