Establishing reaction networks in the 16-electron sulfur reduction reaction
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
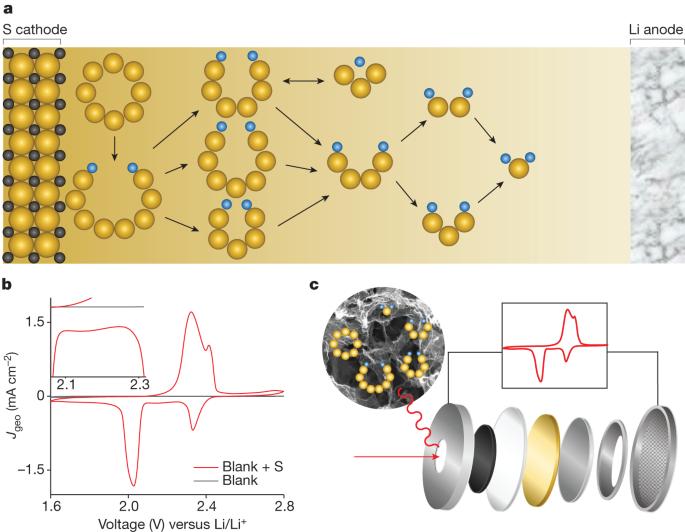
The sulfur reduction reaction (SRR) plays a central role in high-capacity lithium sulfur (Li-S) batteries. The SRR involves an intricate, 16-electron conversion process featuring multiple lithium polysulfide intermediates and reaction branches1–3. Establishing the complex reaction network is essential for rational tailoring of the SRR for improved Li-S batteries, but represents a daunting challenge4–6. Herein we systematically investigate the electrocatalytic SRR to decipher its network using the nitrogen, sulfur, dual-doped holey graphene framework as a model electrode to understand the role of electrocatalysts in acceleration of conversion kinetics. Combining cyclic voltammetry, in situ Raman spectroscopy and density functional theory calculations, we identify and directly profile the key intermediates (S8, Li2S8, Li2S6, Li2S4 and Li2S) at varying potentials and elucidate their conversion pathways. Li2S4 and Li2S6 were predominantly observed, in which Li2S4 represents the key electrochemical intermediate dictating the overall SRR kinetics. Li2S6, generated (consumed) through a comproportionation (disproportionation) reaction, does not directly participate in electrochemical reactions but significantly contributes to the polysulfide shuttling process. We found that the nitrogen, sulfur dual-doped holey graphene framework catalyst could help accelerate polysulfide conversion kinetics, leading to faster depletion of soluble lithium polysulfides at higher potential and hence mitigating the polysulfide shuttling effect and boosting output potential. These results highlight the electrocatalytic approach as a promising strategy for tackling the fundamental challenges regarding Li-S batteries. We investigate the mechanism underlying the sulfur reduction reaction that plays a central role in high-capacity lithium sulfur batteries, highlighting the electrocatalytic approach as a promising strategy for tackling the fundamental challenges associated with these batteries.
在 16 电子硫还原反应中建立反应网络
硫还原反应(SRR)在高容量锂硫(Li-S)电池中发挥着核心作用。硫还原反应涉及一个复杂的 16 电子转换过程,具有多个多硫化锂中间体和反应分支1,2,3。建立复杂的反应网络对于合理调整 SRR 以改进锂-S 电池至关重要,但这也是一项艰巨的挑战4,5,6。在此,我们以氮、硫双掺杂孔状石墨烯框架为模型电极,系统地研究了电催化 SRR,以破译其网络,从而了解电催化剂在加速转化动力学中的作用。结合循环伏安法、原位拉曼光谱和密度泛函理论计算,我们识别并直接剖析了不同电位下的关键中间产物(S8、Li2S8、Li2S6、Li2S4 和 Li2S),并阐明了它们的转化途径。观察到的主要是 Li2S4 和 Li2S6,其中 Li2S4 是决定整个 SRR 动力学的关键电化学中间体。通过配比(歧化)反应生成(消耗)的 Li2S6 不直接参与电化学反应,但在多硫化物穿梭过程中起着重要作用。我们发现,氮、硫双掺杂的孔状石墨烯框架催化剂有助于加速多硫化物的转化动力学,从而在更高的电位下更快地耗尽可溶性多硫化锂,从而减轻多硫化物穿梭效应并提高输出电位。这些结果突出表明,电催化方法是应对锂-S 电池基本挑战的一种有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


