Translating p53-based therapies for cancer into the clinic
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
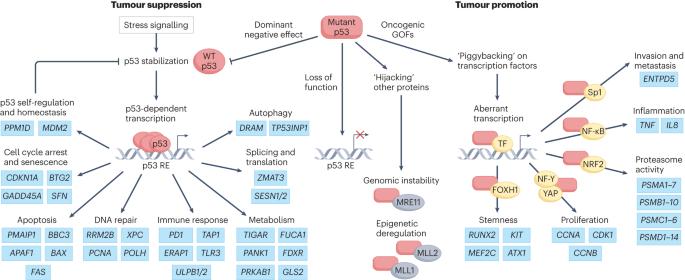
Inactivation of the most important tumour suppressor gene TP53 occurs in most, if not all, human cancers. Loss of functional wild-type p53 is achieved via two main mechanisms: mutation of the gene leading to an absence of tumour suppressor activity and, in some cases, gain-of-oncogenic function; or inhibition of the wild-type p53 protein mediated by overexpression of its negative regulators MDM2 and MDMX. Because of its high potency as a tumour suppressor and the dependence of at least some established tumours on its inactivation, p53 appears to be a highly attractive target for the development of new anticancer drugs. However, p53 is a transcription factor and therefore has long been considered undruggable. Nevertheless, several innovative strategies have been pursued for targeting dysfunctional p53 for cancer treatment. In mutant p53-expressing tumours, the predominant strategy is to restore tumour suppressor function with compounds acting either in a generic manner or otherwise selective for one or a few specific p53 mutations. In addition, approaches to deplete mutant p53 or to target vulnerabilities created by mutant p53 expression are currently under development. In wild-type p53 tumours, the major approach is to protect p53 from the actions of MDM2 and MDMX by targeting these negative regulators with inhibitors. Although the results of at least some clinical trials of MDM2 inhibitors and mutant p53-restoring compounds are promising, none of the agents has yet been approved by the FDA. Alternative strategies, based on a better understanding of p53 biology, the mechanisms of action of compounds and treatment regimens as well as the development of new technologies are gaining interest, such as proteolysis-targeting chimeras for MDM2 degradation. Other approaches are taking advantage of the progress made in immune-based therapies for cancer. In this Review, we present these ongoing clinical trials and emerging approaches to re-evaluate the current state of knowledge of p53-based therapies for cancer. Although p53 was once considered undruggable, in this Review, Peuget et al. discuss the progress made in targeting p53 as a form of cancer therapy with approaches ranging from restoration of mutant p53 function to inhibition of the negative regulator of p53, MDM2, as well as newer strategies, including p53-based mRNA vaccines and antibodies.
将基于 p53 的癌症疗法应用于临床。
最重要的肿瘤抑制基因 TP53 失活发生在大多数(如果不是全部)人类癌症中。野生型 p53 功能的丧失主要通过两种机制实现:基因突变导致肿瘤抑制活性的缺失,在某些情况下还会导致致癌功能的获得;或者野生型 p53 蛋白因其负性调节因子 MDM2 和 MDMX 的过度表达而受到抑制。由于 p53 具有很强的肿瘤抑制作用,而且至少某些已确诊的肿瘤依赖于它的失活,因此它似乎是开发新抗癌药物的一个极具吸引力的靶点。然而,p53 是一种转录因子,因此长期以来一直被认为是不可药用的。尽管如此,针对功能失调的 p53 治疗癌症的一些创新策略仍在研究之中。在表达突变 p53 的肿瘤中,最主要的策略是通过以通用方式或针对一种或几种特定 p53 突变进行选择性作用的化合物来恢复肿瘤抑制功能。此外,目前还在开发清除突变 p53 或针对突变 p53 表达所造成的脆弱性的方法。在野生型 p53 肿瘤中,主要的方法是通过抑制剂靶向 MDM2 和 MDMX 这些负调控因子来保护 p53 免受 MDM2 和 MDMX 的作用。虽然至少一些 MDM2 抑制剂和突变 p53 恢复化合物的临床试验结果令人鼓舞,但这些药物都尚未获得美国食品及药物管理局的批准。基于对 p53 生物学、化合物的作用机制和治疗方案的更好理解,以及新技术的开发,替代策略正受到越来越多的关注,例如用于降解 MDM2 的蛋白水解靶向嵌合体。其他方法也在利用基于免疫的癌症疗法所取得的进展。在本综述中,我们将介绍这些正在进行的临床试验和新出现的方法,以重新评估基于 p53 的癌症疗法的知识现状。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


