The Swiss Spinal Cord Injury Cohort Study (SwiSCI) biobank: from concept to reality
IF 2.2
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
Abstract
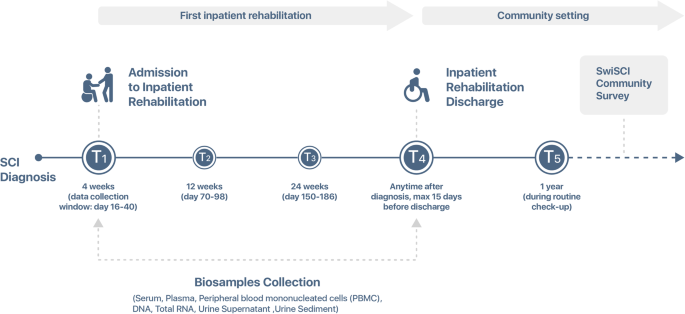
To describe the concept, establishment and the operationalization of the biobank of the Swiss Spinal Cord Injury Cohort Study (SwiSCI), the available biosamples, and demographic and clinical characteristics of study participants. The SwiSCI biobank is a platform for research within SwiSCI. It collects and processes serum, plasma, PBMCs, RNA, DNA, and urine from three rehabilitation centers. Samples are collected at admission to first rehabilitation and at discharge. Additionly, the biobank provides services to projects nested in SwiSCI or otherclinical trials among Spinal Cord Injury population. Descriptive statistics were used for an overview of available biosamples, study participant characteristics, and comparison of the participating centers. Between the SwiSCI biobank establishment on June 27th, 2016, and October 19th, 2023, the SwiSCI Study has obtained informed consent from 524 individuals. Of these, 315 (60.1%) have agreed to donate biospecimens to the biobank. The average age of the contributors was 54 years (range: 38–65), with the majority being male (80%). Most participants suffered from traumatic injuries (66%) and were classified as paraplegic (64%). Approximately 80% presented with motor and sensory-incomplete SCI. The median Spinal Cord Independence Measure (SCIM) score was 31 (Interquartile Range: 19–58). The proportion of individuals providing paired biosamples at two distinct time points ranged from 63% (for RNA) to 65% (for urine and urine sediment). The SwiSCI biobank is a unique platform designed to serve as a basis for collaborative SCI research, including multi-omics approaches. The longitudinal collection of biospecimens and cryopreservation of multiple aliquots for each participant are fundamental for scrutinizing the temporal associations, ensuring research reproducibility, and achieving an adequate sample size for future investigations.
瑞士脊髓损伤队列研究(SwiSCI)生物库:从概念到现实。
研究目的描述瑞士脊髓损伤队列研究(SwiSCI)生物库的概念、建立和运作情况、可用的生物样本以及研究参与者的人口和临床特征:瑞士脊髓损伤队列研究生物库是瑞士脊髓损伤队列研究的一个研究平台。它收集并处理来自三个康复中心的血清、血浆、PBMCs、RNA、DNA 和尿液。样本在首次康复入院和出院时收集。此外,生物库还为脊髓损伤人群中的 SwiSCI 嵌套项目或其他临床试验提供服务:方法:使用描述性统计概述可用生物样本、研究参与者特征以及参与中心的比较:从 2016 年 6 月 27 日 SwiSCI 生物库建立到 2023 年 10 月 19 日,SwiSCI 研究已获得 524 人的知情同意。其中,315 人(60.1%)同意向生物库捐献生物样本。捐献者的平均年龄为 54 岁(38-65 岁不等),大多数为男性(80%)。大多数参与者都受过外伤(66%),并被归类为截瘫患者(64%)。约 80% 的人患有运动和感觉不完全 SCI。脊髓独立性测量(SCIM)的中位数为 31 分(四分位间范围:19-58)。在两个不同时间点提供配对生物样本的患者比例从63%(RNA)到65%(尿液和尿沉渣)不等:SwiSCI生物样本库是一个独特的平台,旨在作为SCI合作研究的基础,包括多组学方法。纵向收集生物样本并对每位参与者的多个等分样本进行冷冻保存,对于仔细研究时间关联、确保研究的可重复性以及为未来的调查获得足够的样本量至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


