Synthesis of 1,4‐Dihydropyridines via Addition of Furfural‐Derived Trienolates to Pyridiniums under NHC Catalysis
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
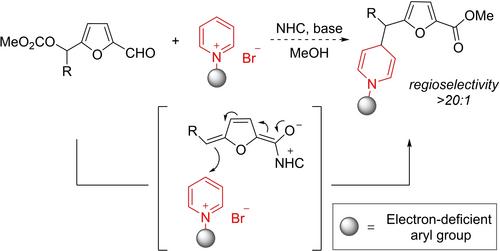
Both furan and 1,4‐dihydropyridine (1,4‐DHP) are key structural motifs in pharmaceuticals. Herein, we report the synthesis of 4‐(furan‐2‐ylmethyl)‐1,4‐dihydropyridines from pyridiniums and furfural derivatives under NHC catalysis. This reaction involves the generation of NHC‐bounded trienolates from furfural derivatives and following addition to the C‐4 position of N‐aryl pyridinium salts. This protocol features good yields (41–99%), and high levels of regioselectivity (>20:1 for all cases).
在 NHC 催化下通过糠醛衍生的三苯酚与吡啶鎓的加成合成 1,4-二氢吡啶类化合物
呋喃和 1,4-二氢吡啶(1,4-DHP)都是药物中的关键结构基团。在此,我们报告了在 NHC 催化下从吡啶鎓和糠醛衍生物合成 4-(呋喃-2-基甲基)-1,4-二氢吡啶的过程。该反应包括从糠醛衍生物中生成 NHC 键合的三烯酚,然后加到 N-芳基吡啶鎓盐的 C-4 位置。该方案具有良好的产率(41-99%)和高水平的区域选择性(所有情况下均为 20:1)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


