Horizontal gene transfer in eukaryotes: aligning theory with data
IF 39.1
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
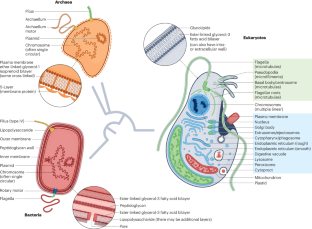
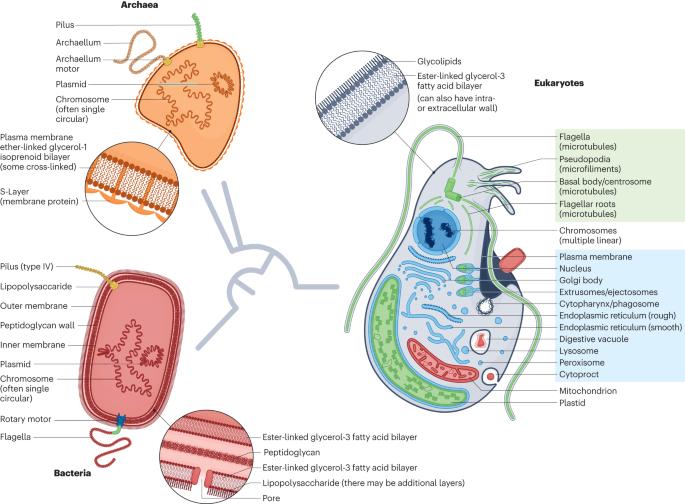
Horizontal gene transfer (HGT), or lateral gene transfer, is the non-sexual movement of genetic information between genomes. It has played a pronounced part in bacterial and archaeal evolution, but its role in eukaryotes is less clear. Behaviours unique to eukaryotic cells — phagocytosis and endosymbiosis — have been proposed to increase the frequency of HGT, but nuclear genomes encode fewer HGTs than bacteria and archaea. Here, I review the existing theory in the context of the growing body of data on HGT in eukaryotes, which suggests that any increased chance of acquiring new genes through phagocytosis and endosymbiosis is offset by a reduced need for these genes in eukaryotes, because selection in most eukaryotes operates on variation not readily generated by HGT. In this Review, Patrick Keeling proposes that the eukaryotic-specific processes of phagocytosis and endosymbiosis are unlikely to increase the frequency of horizontal gene transfers, because most of the transferred genes will be non-essential and will thus not be selected for the long term.
真核生物中的水平基因转移:理论与数据的统一
水平基因转移(HGT)或横向基因转移是基因组之间遗传信息的非有性移动。它在细菌和古细菌的进化过程中发挥了显著作用,但在真核生物中的作用却不那么明显。真核细胞特有的行为--吞噬作用和内共生--被认为会增加HGT的频率,但与细菌和古细菌相比,核基因组编码的HGT较少。在此,我将结合越来越多的真核生物 HGT 数据对现有理论进行回顾,这些数据表明,通过吞噬作用和内共生作用获得新基因的几率增加,但真核生物对这些基因的需求减少,从而抵消了增加的几率,因为大多数真核生物的选择作用是针对不容易通过 HGT 产生的变异进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Genetics
生物-遗传学
CiteScore
57.40
自引率
0.50%
发文量
113
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Genetics, our goal is to be the leading source of reviews and commentaries for the scientific communities we serve. We are dedicated to publishing authoritative articles that are easily accessible to our readers. We believe in enhancing our articles with clear and understandable figures, tables, and other display items. Our aim is to provide an unparalleled service to authors, referees, and readers, and we are committed to maximizing the usefulness and impact of each article we publish.
Within our journal, we publish a range of content including Research Highlights, Comments, Reviews, and Perspectives that are relevant to geneticists and genomicists. With our broad scope, we ensure that the articles we publish reach the widest possible audience.
As part of the Nature Reviews portfolio of journals, we strive to uphold the high standards and reputation associated with this esteemed collection of publications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


