Haematopoietic innate interleukin 17A production drives immunopathology in female mouse genital Chlamydia muridarum infection
IF 4.1
4区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
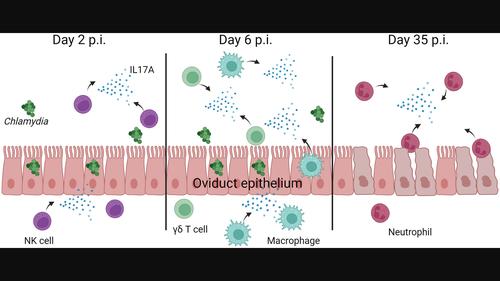
Chlamydia trachomatis infection is the leading cause of bacterial urogenital infection and has been demonstrated to drive inflammation and scarring of the reproductive tract. Recent studies have identified key triggers of proinflammatory adaptive immune responses driven by innate leukocytes and epithelia driving immunopathology. Utilizing chimeric mouse models, we investigated the definitive source and role of IL17 and IL17 signalling receptors during early Chlamydia muridarum infection of the female urogenital tract. Bone marrow transplants from wild-type (WT) and IL17A−/− mice to recipients demonstrated equivocal infection kinetics in the reproductive tract, but interestingly, adoptive transfer of IL17A−/− immune cells to WT recipients resulted in no infertility, suggesting a haematopoietic (as opposed to tissue) source of IL17 driving immunopathology. To further delineate the role of IL17 in immunopathology, we infected WT and IL17 receptor A (IL17RA)−/− female mice and observed a significant reduction in immunopathology in IL17RA−/− mice. WT bone marrow transplants to IL17RA−/− recipient mice prevented hydrosalpinx, suggesting signalling through IL17RA drives immunopathology. Furthermore, early chemical inhibition of IL17 signalling significantly reduced hydrosalpinx, suggesting IL17 acts as an innate driver of disease. Early during the infection, IL17 was produced by γδ T cells in the cervico-vagina, but more importantly, by neutrophils at the site of infertility in the oviducts. Taken together, these data suggest innate production of IL17 by haematopoietic leukocytes drives immunopathology in the epithelia during early C. muridarum infection of the female reproductive tract.
造血先天性白细胞介素 17A 的产生推动了雌性小鼠生殖器衣原体感染的免疫病理学发展
沙眼衣原体感染是细菌性泌尿生殖道感染的主要病因,已被证实可导致生殖道炎症和瘢痕形成。最近的研究发现,先天性白细胞和上皮细胞驱动的促炎性适应性免疫反应是导致免疫病理的关键诱因。利用嵌合小鼠模型,我们研究了IL17和IL17信号受体在女性泌尿生殖道早期衣原体感染过程中的明确来源和作用。将野生型(WT)和IL17A-/-小鼠的骨髓移植给受体后,生殖道的感染动力学表现不一,但有趣的是,将IL17A-/-免疫细胞收养性转移给WT受体不会导致不孕,这表明驱动免疫病理学的IL17来源于造血(而非组织)。为了进一步明确IL17在免疫病理学中的作用,我们感染了WT和IL17受体A(IL17RA)-/-雌性小鼠,观察到IL17RA-/-小鼠的免疫病理学显著降低。将 WT 骨髓移植给 IL17RA-/- 受体小鼠可防止水肿,这表明通过 IL17RA 的信号驱动了免疫病理学。此外,早期化学抑制 IL17 信号可显著减少水肿,表明 IL17 是疾病的先天驱动因素。在感染早期,宫颈阴道中的γδT细胞产生了IL17,但更重要的是,输卵管不孕部位的中性粒细胞也产生了IL17。总之,这些数据表明,造血白细胞先天性产生的 IL17 驱动了女性生殖道早期鼠疫感染时上皮细胞的免疫病理学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
5.40%
发文量
109
审稿时长
1 months
期刊介绍:
This peer-reviewed international journal publishes original articles and reviews on all aspects of basic, translational and clinical immunology. The journal aims to provide high quality service to authors, and high quality articles for readers.
The journal accepts for publication material from investigators all over the world, which makes a significant contribution to basic, translational and clinical immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


