Extraction of rare earth elements from neodymium (NdFeB) magnet scrap using magnesium halides
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The demand for neodymium (NdFeB) permanent magnets for electric vehicles and eco-friendly generators is increasing. However, NdFeB magnets contain rare earth elements (REEs), which are limited in supply. In this study, we performed an exchange reaction between magnesium halides (fluoride and chloride) and waste NdFeB scrap and then compared the characteristics of the extracted halides salts. The compositions of the ternary Mg fluoride (LiF:NaF:MgF2 = 50:40:10 in mole ratio) and chloride (LiCl:NaCl:MgCl2 = 10:50:40 in mole ratio) salts were thermodynamically determined for achieving low eutectic temperatures. The reactions between the NdFeB scrap powder (1–2 mm) and Mg halide salts were carried out at 1073 and 873 K for the fluoride and chloride systems, respectively, in an argon atmosphere. After the reaction, we separated Nd halide from the residual salt and evaluated the Nd-extraction rate. The phase formation of the salt was analyzed using X-ray diffraction (XRD), and the extraction rate of Nd was analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES). Nd was extracted in the form of Nd halide (NdF3 or NdCl3), and the extraction rates in the fluoride and chloride systems are 98.64% and 84.59%, respectively. Thus, the fluoride system is more effective than the chloride system for Nd extraction. Our study provides a comprehensive comparative analysis of the effectiveness of fluoride and chloride systems in extracting REEs from NdFeB magnet scrap. Our study findings can be used to develop an effective method for recycling magnet scraps.
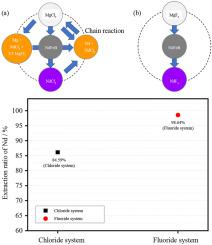

利用卤化镁从钕铁硼(NdFeB)磁铁废料中提取稀土元素
电动汽车和环保发电机对钕(NdFeB)永磁体的需求日益增长。然而,钕铁硼磁体含有稀土元素,而稀土元素的供应有限。在本研究中,我们在卤化镁(氟化物和氯化物)和废弃钕铁硼废料之间进行了交换反应,然后比较了提取的卤化物盐的特性。通过热力学方法确定了氟化镁(LiF:NaF:MgF2 = 50:40:10,摩尔比)和氯化镁(LiCl:NaCl:MgCl2 = 10:50:40,摩尔比)三元盐的组成,以实现低共晶温度。钕铁硼废料粉末(1-2 毫米)与卤化镁盐之间的反应是在氩气环境中分别于 1073 和 873 K 的温度下进行的。反应结束后,我们从残盐中分离出卤化钕,并评估了钕萃取率。使用 X 射线衍射 (XRD) 分析了盐的相形成,并使用电感耦合等离子体光发射光谱 (ICP-OES) 分析了钕的萃取率。钕以卤化钕(NdF3 或 NdCl3)的形式被萃取,氟化物和氯化物体系的萃取率分别为 98.64 % 和 84.59 %。因此,在萃取钕方面,氟化物体系比氯化物体系更有效。我们的研究对氟化物和氯化物体系从钕铁硼磁铁废料中提取稀土元素的有效性进行了全面的比较分析。我们的研究结果可用于开发一种回收磁铁废料的有效方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


