Metabolic alterations in hereditary and sporadic renal cell carcinoma
IF 28.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Kidney cancer is the seventh leading cause of cancer in the world, and its incidence is on the rise. Renal cell carcinoma (RCC) is the most common form and is a heterogeneous disease comprising three major subtypes that vary in their histology, clinical course and driver mutations. These subtypes include clear cell RCC, papillary RCC and chromophobe RCC. Molecular analyses of hereditary and sporadic forms of RCC have revealed that this complex and deadly disease is characterized by metabolic pathway alterations in cancer cells that lead to deregulated oxygen and nutrient sensing, as well as impaired tricarboxylic acid cycle activity. These metabolic changes facilitate tumour growth and survival. Specifically, studies of the metabolic features of RCC have led to the discovery of oncometabolites — fumarate and succinate — that can promote tumorigenesis, moonlighting functions of enzymes, and substrate auxotrophy owing to the disruption of pathways that enable the production of arginine and cholesterol. These metabolic alterations within RCC can be exploited to identify new therapeutic targets and interventions, in combination with novel approaches that minimize the systemic toxicity of metabolic inhibitors and reduce the risk of drug resistance owing to metabolic plasticity. Renal cell carcinoma is a metabolic disease linked to a variety of alterations in genes that regulate cellular metabolism. Here, the authors examine cell-intrinsic metabolic alterations in hereditary and sporadic renal cell carcinoma, and how they can be exploited to develop novel therapeutic interventions.
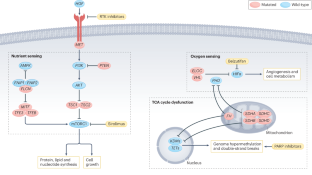
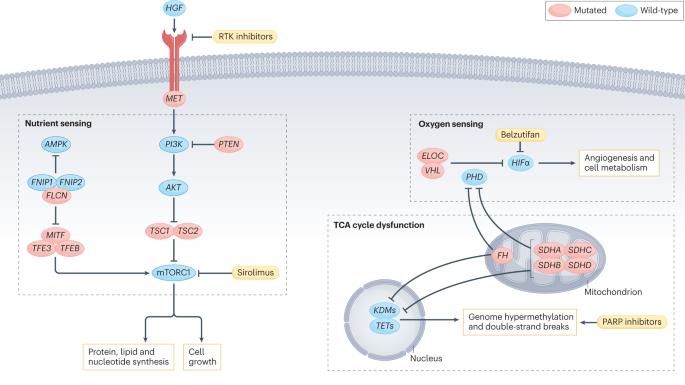
遗传性和散发性肾细胞癌的代谢改变
肾癌是全球第七大癌症,且发病率呈上升趋势。肾细胞癌(RCC)是最常见的癌症,也是一种异质性疾病,包括三大亚型,它们在组织学、临床病程和驱动基因突变方面各不相同。这些亚型包括透明细胞 RCC、乳头状 RCC 和嗜铬细胞 RCC。对遗传性和散发性 RCC 进行的分子分析表明,这种复杂而致命的疾病的特点是癌细胞的代谢途径发生改变,导致氧和营养感应失调以及三羧酸循环活性受损。这些代谢变化促进了肿瘤的生长和存活。具体来说,通过对 RCC 代谢特征的研究,发现了可促进肿瘤发生的副代谢物--富马酸盐和琥珀酸盐、酶的 "月光 "功能,以及由于精氨酸和胆固醇生成途径被破坏而导致的底物辅助营养。可以利用 RCC 内部的这些代谢改变来确定新的治疗靶点和干预措施,并结合新的方法,最大限度地减少代谢抑制剂的全身毒性,降低因代谢可塑性而产生耐药性的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


